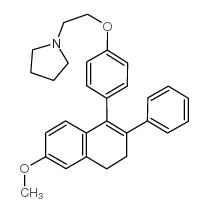
Nafoxidine HCl
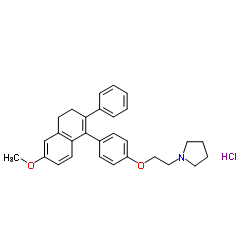
Nafoxidine HCl structure
|
Common Name | Nafoxidine HCl | ||
|---|---|---|---|---|
| CAS Number | 1847-63-8 | Molecular Weight | 462.023 | |
| Density | 1.137g/cm3 | Boiling Point | 574.5ºC at 760mmHg | |
| Molecular Formula | C29H32ClNO2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 176.7ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
Use of Nafoxidine HClNafoxidine, also known as PNU-0011100 and CP-5600, is a partial estrogen antagonist. Nafoxidine competes with endogenous estrogen for binding to specific estrogen receptors. This agent also inhibits angiogenesis in some tissues by blocking the effects of fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF); paradoxically, it may enhance angiogenesis in uterine tissue. Nafoxidine also induces oxidative stress, protein kinase C and calcium signaling. |
| Name | Nafoxidine hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Density | 1.137g/cm3 |
|---|---|
| Boiling Point | 574.5ºC at 760mmHg |
| Molecular Formula | C29H32ClNO2 |
| Molecular Weight | 462.023 |
| Flash Point | 176.7ºC |
| Exact Mass | 461.212158 |
| PSA | 21.70000 |
| LogP | 6.81500 |
| Vapour Pressure | 3.33E-13mmHg at 25°C |
| Index of Refraction | 1.608 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H312-H351 |
| Precautionary Statements | P280 |
| Hazard Codes | Xi |
| RIDADR | UN 2811 |
| RTECS | UY0880000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
|
~% 
Nafoxidine HCl CAS#:1847-63-8 |
| Literature: US2005/70512 A1, ; Page/Page column 23 ; |
|
~% 
Nafoxidine HCl CAS#:1847-63-8 |
| Literature: WO2010/125578 A2, ; Page/Page column 20; 21 ; |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
|
Prediction of ligand binding affinity using a multiple-conformations-multiple-protonation scheme: application to estrogen receptor α.
Chem. Pharm. Bull. 60(2) , 183-94, (2012) A fast method that can predict the binding affinities of chemicals to a target protein with a high degree of accuracy will be very useful in drug design and regulatory science. We have been developing... |
|
|
Tamoxifen and related compounds protect against lipid peroxidation in isolated nuclei: relevance to the potential anticarcinogenic benefits of breast cancer prevention and therapy with tamoxifen?
Free Radic. Biol. Med. 17(5) , 485-8, (1994) Tamoxifen, 4-hydroxytamoxifen, nafoxidine, 17 beta-oestradiol and ICI 164,384 were all found to protect rat liver nuclei against Fe(III)-ascorbate dependent lipid peroxidation. The order of effectiven... |
|
|
Multifactorial activities of nonsteroidal antiestrogens against leukemia.
Cancer Detect. Prev. 27(5) , 389-96, (2003) The antileukemic activity of nonsteroidal antiestrogens was investigated. Tamoxifen, clomiphene and nafoxidine caused a decrease in viability of the estrogen receptor-negative T-lymphoblastic leukemia... |
| Nafoxidine Hydrochloride |
| Pyrrolidine, 1-[2-[4-(3,4-dihydro-6-methoxy-2-phenyl-1-naphthalenyl)phenoxy]ethyl]-, hydrochloride (1:1) |
| Pyrrolidine 1-[2-[4-(3,4-dihydro-6-methoxy-2-phenyl-1-naphthalenyl)phenoxy]ethyl]-, hydrochloride |
| Nafoxidine HCl |
| 1-{2-[4-(6-Methoxy-2-phenyl-3,4-dihydro-1-naphthalenyl)phenoxy]ethyl}pyrrolidine hydrochloride (1:1) |
![1-{2-[4-(2-BROMO-6-METHOXY-3,4-DIHYDRO-1-NAPHTHYL)PHENOXY]ETHYL}PYRROLIDINE structure](https://image.chemsrc.com/caspic/241/180915-95-1.png)