N-(2-Oxotetrahydrofuran-3-yl)dodecanamide
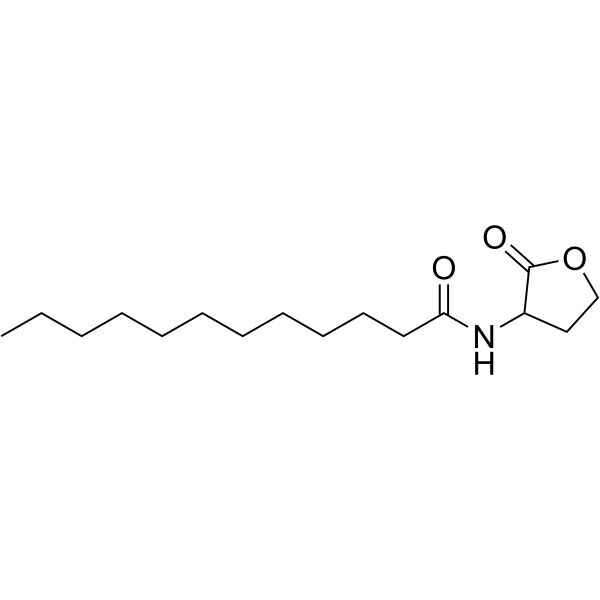
N-(2-Oxotetrahydrofuran-3-yl)dodecanamide structure
|
Common Name | N-(2-Oxotetrahydrofuran-3-yl)dodecanamide | ||
|---|---|---|---|---|
| CAS Number | 18627-38-8 | Molecular Weight | 283.40600 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C16H29NO3 | Melting Point | 114-117 ℃ | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of N-(2-Oxotetrahydrofuran-3-yl)dodecanamideN-Dodecanoyl-DL-homoserine lactone is a serine derivative[1]. |
| Name | n-dodecanoyl-dl-homoserine lactone |
|---|---|
| Synonym | More Synonyms |
| Description | N-Dodecanoyl-DL-homoserine lactone is a serine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Melting Point | 114-117 ℃ |
|---|---|
| Molecular Formula | C16H29NO3 |
| Molecular Weight | 283.40600 |
| Exact Mass | 283.21500 |
| PSA | 55.40000 |
| LogP | 3.72990 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2932209090 |
| HS Code | 2932209090 |
|---|---|
| Summary | 2932209090. other lactones. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Zoospore interspecific signaling promotes plant infection by Phytophthora.
BMC Microbiol. 10 , 313, (2010) Oomycetes attack a huge variety of economically and ecologically important plants. These pathogens release, detect and respond to signal molecules to coordinate their communal behaviors including the ... |
|
|
Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones.
Microbiology 143 , 3703, (1997) Quorum sensing relies upon the interaction of a diffusible signal molecule with a transcriptional activator protein to couple gene expression with cell population density. In Gram-negative bacteria, s... |
|
|
Quorum sensing in the genus Burkholderia.
Int. J. Med. Microbiol. 296 , 103-110, (2006) The genus Burkholderia contains over 30 species, many of which are important human pathogens. In addition to the primary pathogens Burkholderia pseudomallei and Burkholderia mallei, several species ha... |
| N-dodecanoyl-HSL |
| N-dodecanoyl-DL-homoserine |
| 3-dodecanoylamino-dihydro-furan-2-one |
| n-lauroyl-dl-homoserine lactone |
| N-Dodecanoyl-DL-homoserine lactone |
| N-dodecanoyl-homoserine lactone |