| Description |
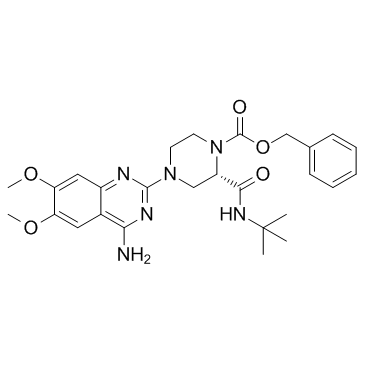
L-765314 is a potent and selective α1b adrenergic receptor antagonist with Kis of 5.4 nM and 2.0 nM for rat and human α1b adrenergic receptor, respectively.
|
| Related Catalog |
|
| Target |
Ki: 5.4±0.6 nM (rat α1b receptor ), 2.0±0.66 nM (human α1b receptor), 50±8 nM (rat α1d receptor), 34±6 nM (human α1d receptor), 500±20 nM (rat α1b receptor ), 420±62 nM (human α1b receptor)[1].
|
| In Vitro |
L-765314 exhibits two displacement sites. The high-affinity site accounts for approximately 25% of binding (IC50) 1.90 nM and represents binding to the R1b sites. The low-affinity site accounts for the residual 75% of binding (IC50) 790 nM and represents binding to the R1a sites[1].
|
| In Vivo |
The results of plasma assayed by liquid chromatograph/mass spectrometer (LCMS) show that the mean Cmax of L-765314 (A322312) is 1.05 μM and the t1/2 is 0.5 h. L-765314 shows weak potency for inhibiting the pressor response to either phenylephrine or A-61603 (AD25>3 mg/kg for each). On the basis of the inhibition of pressor responses to the R1a subtype selective agonist A-61603, L-765314 appears to be selective versus the R1a receptor up to a dose of 0.3 mg/kg. The results of hypotensive potency in rats show that both L-765314 and terazosin tend to decrease heart rate (about 25 bpm at 1 mg/kg iv)[1].
|
| Animal Admin |
Rats[1] The potency of terazosin and L-765314 for inhibiting the pressor responses to phenylephrine and A-61603 is evaluated in anesthetized male Sprague-Dawley rats (n=4). The rats are dosed i.v with either vehicle or ascending doses of test compounds, and the peak changes in mean arterial pressure are measured. The dose of antagonist eliciting a 25 mmHg decrease in mean arterial pressure (AD25) is calculated as an index of hypotensive potency. The rats are dosed i.v with L-765314 at 3 mg/kg , and the plasma is assayed by LCMS for parent compound[1].
|
| References |
[1]. Patane MA, et al. 4-Amino-2-[4-[1-(benzyloxycarbonyl)-2(S)- [[(1,1-dimethylethyl)amino]carbonyl]-piperazinyl]-6, 7-dimethoxyquinazoline (L-765,314): a potent and selective alpha1b adrenergic receptor antagonist. J Med Chem. 1998 Apr 9;41(8):1205-8. [2]. Tobias Böhmer, et al. The α1B-adrenoceptor subtype mediates adrenergic vasoconstriction in mouse retinal arterioles with damaged endothelium. Br J Pharmacol. 2014 Aug; 171(16): 3858–3867.
|