3,3'-Diindolylmethane
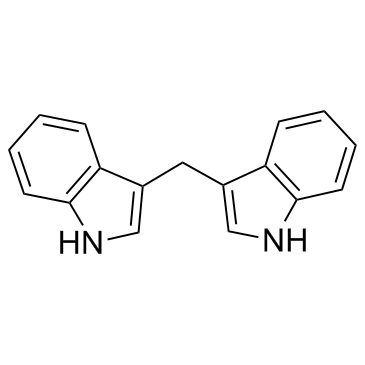
3,3'-Diindolylmethane structure
|
Common Name | 3,3'-Diindolylmethane | ||
|---|---|---|---|---|
| CAS Number | 1968-05-4 | Molecular Weight | 246.307 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 504.8±30.0 °C at 760 mmHg | |
| Molecular Formula | C17H14N2 | Melting Point | 167 °C | |
| MSDS | USA | Flash Point | 232.5±15.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 3,3'-Diindolylmethane3,3'-Diindolylmethane is a strong, pure androgen receptor (AR) antagonist. |
| Name | 3,3'-diindolylmethane |
|---|---|
| Synonym | More Synonyms |
| Description | 3,3'-Diindolylmethane is a strong, pure androgen receptor (AR) antagonist. |
|---|---|
| Related Catalog | |
| Target |
Androgen receptor[1] |
| In Vitro | 3,3'-Diindolylmethane (DIM) is a strong antagonist of androgen receptor (AR) function but exhibits less than obvious structural similarity to the endogenous AR ligand, dihydrotestosterone (DHT). 3,3'-Diindolylmethane is a major digestive product of indole-3-carbinol, a potential anticancer component of cruciferous vegetables. 3,3'-Diindolylmethane exhibits potent antiproliferative and antiandrogenic properties in androgen-dependent human prostate cancer cells. 3,3'-Diindolylmethane suppresses cell proliferation of LNCaP cells and inhibits DHT stimulation of DNA synthesis. Moreover, 3,3'-Diindolylmethane inhibits endogenous PSA transcription and reduced intracellular and secreted PSA protein levels induced by DHT in LNCaP cells. Also, 3,3'-Diindolylmethane inhibits, in a concentration-dependent manner, the DHT-induced expression of a prostate-specific antigen promoter-regulated reporter gene construct in transiently transfected LNCaP cells. Co-treatment with 50 μM 3,3'-Diindolylmethane partially inhibits the translocation of AR induced by DHT treatment and showed distribution of the AR to be both cytoplasmic and nuclear. Furthermore, 3,3'-Diindolylmethane treatment prevents the formation of AR foci in the nucleus. 3,3'-Diindolylmethane alone produces a predominantly cytoplasmic distribution of fluorescence[1]. |
| In Vivo | Mice are randomized into two groups and are treated daily s.c. with either vehicle or 3,3'-Diindolylmethane (10 mg/kg) for 30 days. Tumor volume and the weight of mice are recorded once every 3 days using calipers. 3,3'-Diindolylmethane (DIM) treatment resulted in a marked inhibition of SNU-484 xenograft tumor growth. Notably, the body weight of mice from both groups did not significantly differ from the vehicle control following 30 days of drug exposure, suggesting that 3,3'-Diindolylmethane has no severe toxicity to the mice. Taken together, these findings demonstrate that 3,3'-Diindolylmethane administration significantly inhibited SNU-484 xenograft growthin vivo mediated by the inactivation of YAP[2]. |
| Cell Assay | The human prostate adenocarcinoma cell lines LNCaP-FGC and PC-3 are grown as adherent monolayers in 10% FBS-DMEM, supplemented with 4.0 g/L glucose and 3.7 g/L sodium bicarbonate in a humidified incubator at 37°C and 5% CO2, and passaged at ~80% confluency. Cultures used in subsequent experiments are at less than 40 passages. Cells grown in stripped conditions are in 5% DCC-FBS-DMEM base supplemented with 4 g/L glucose, 3.7 g/L sodium bicarbonate, and 0.293 g/L L-glutamine. Before the beginning of the treatments, cells are depleted of androgen for 4-7 days in medium composed of DMEM base without phenol red and with 4 g/L glucose and 3.7 g/L sodium bicarbonate. During the depletion period, medium is changed every 48 h. Treatments are administered by the addition of 1 μL of a 1,000-fold concentrated solution of 3,3'-Diindolylmethane in Me2SO/mL of medium. Once the treatment period started, medium is changed daily to counter possible loss of readily metabolized compounds[1]. |
| Animal Admin | Mice[2] Four-week-old female SPF/VAF immunodeficient mice are injected subcutaneously (s.c.) into the right flank with 0.1 mL Matrigel containing 3.5×106 human gastric cancer cells (SNU-484). The mice are randomized into 2 groups 1 week after tumor implantation: i) the untreated control group (n=5, DMSO in 50 μL PBS daily) and ii) the 3,3'-Diindolylmethane-treated group (n=5, 10 mg/kg in 50 μL PBS once daily). Gastric primary tumors are excised, and the final tumor volume is measured once every 3 days using a caliper and calculated as (width)2×length/2. The experiment is terminated on day 39. Half of the tumor tissue is prepared for western blotting and the other half is snap frozen in liquid nitrogen and stored at −80°C. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 504.8±30.0 °C at 760 mmHg |
| Melting Point | 167 °C |
| Molecular Formula | C17H14N2 |
| Molecular Weight | 246.307 |
| Flash Point | 232.5±15.8 °C |
| Exact Mass | 246.115692 |
| PSA | 31.58000 |
| LogP | 4.05 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.765 |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335-H413 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| RTECS | NM0332000 |
| HS Code | 2933990090 |
| Precursor 10 | |
|---|---|
| DownStream 3 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Actinobacillus pleuropneumoniae induces SJPL cell cycle arrest in G2/M-phase and inhibits porcine reproductive and respiratory syndrome virus replication.
Virol. J. 12 , 188, (2015) Porcine reproductive and respiratory syndrome virus (PRRSV) is one of the most important pathogens in the swine industry and causes important economic losses. No effective antiviral drugs against it a... |
|
|
The extended version of restriction analysis approach for the examination of the ability of low-molecular-weight compounds to modify DNA in a cell-free system.
Food Chem. Toxicol. 75 , 118-27, (2015) One of the primary requirements in toxicology is the assessment of ability of chemicals to induce DNA covalent modification. There are several well-established methods used for this purpose such as (3... |
|
|
The roles of co-chaperone CCRP/DNAJC7 in Cyp2b10 gene activation and steatosis development in mouse livers.
PLoS ONE 9(12) , e115663, (2014) Cytoplasmic constitutive active/androstane receptor (CAR) retention protein (CCRP and also known as DNAJC7) is a co-chaperone previously characterized to retain nuclear receptor CAR in the cytoplasm o... |
| HB 236 |
| 1H-Indole, 2-(1H-indol-3-ylmethyl)- |
| 3-(1H-indol-3-ylmethyl)-1H-indole |
| ARUNDINE |
| di(1H-indol-3-yl)methane |
| DI-3-INDOLYLMETHANE |
| DIM |
| 3-DIINDOLYL METHANE |
| 3,3'-Diindolylmethane |
| 1H-Indole, 3,3'-methylenebis- |
| 2-(1H-Indol-3-ylmethyl)-1H-indole |
| 3,3'-Methylenediindole |
| 3,3'-Methylenebisindole;DIM |
| DI INDOLYL METHANE |
| di-(1H-3-indolyl)methane |
| Indole dimer |
| 3,3'-methylene-bis(1H-indole) |
| 3,3-Methylenediindole |
| Indolyl-CH2-Indolyl |
| MFCD00195766 |
| 3,3'-Methylenebis(1H-indole) |
| 3,3'-Diindolymetane |
| Diindole methane |
 CAS#:120-72-9
CAS#:120-72-9 CAS#:50-00-0
CAS#:50-00-0 CAS#:700-06-1
CAS#:700-06-1 CAS#:110-18-9
CAS#:110-18-9 CAS#:100-97-0
CAS#:100-97-0 CAS#:1373922-48-5
CAS#:1373922-48-5 CAS#:31899-46-4
CAS#:31899-46-4 CAS#:14558-49-7
CAS#:14558-49-7 CAS#:67-56-1
CAS#:67-56-1 CAS#:20503-92-8
CAS#:20503-92-8![5,11-Dihydroindolo[3,2-b]carbazole structure](https://image.chemsrc.com/caspic/092/6336-32-9.png) CAS#:6336-32-9
CAS#:6336-32-9 CAS#:61844-15-3
CAS#:61844-15-3 CAS#:526-32-9
CAS#:526-32-9
