Atazanavir
Modify Date: 2024-01-02 18:25:09

Atazanavir structure
|
Common Name | Atazanavir | ||
|---|---|---|---|---|
| CAS Number | 198904-31-3 | Molecular Weight | 704.856 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C38H52N6O7 | Melting Point | 207-209ºC | |
| MSDS | N/A | Flash Point | N/A | |
Use of AtazanavirAtazanavir(BMS-232632) is an highly potent HIV-1 protease inhibitor.IC50 value:Target: HIV-1 protease inhibitorAtazanavir sulfate is a sulfate salt form of atazanavir that is an highly potent HIV-1 protease inhibitor. It has a pharmacokinetic profile that supports once-daily dosing and has demonstrated a unique resistance profile and superior virologic potency compared with other antiretrovirals in vitro. In subjects with HIV, atazanavir (400 mg once daily) produced rapid and sustained improvements in viral load and CD4 counts in both antiretroviral-naive as well as previously treated patients when used in combination with dual nucleoside reverse transcriptase inhibitor (NRTI) treatment [1].After intravenous (iv), oral (po) and intraportal (ip) administration of ATV at a dosage of 7 mg/kg, AUCs in HL rats were 12.41, 5.24 and 8.89 microg/mLh, respectively, and were significantly higher than those in control rats (4.09, 1.70 and 3.38 microg/mLh). Despite the decrease of distribution volume (Vd(ss)), the terminal half-life (t(1/2)) in HL tended to be shorter than in control, and hepatic distribution of ATV in HL rats was 4.8-fold increases. These results suggested that the uptake of ATV into liver might counteract the decrease of Vd(ss). On the other hand, there was no significant difference in bioavailability, and the lymphatic transport to AUC showed no statistical change. In conclusion, although the protein binding rate and AUC were significantly increased, the pharmacokinetics of ATV might be tolerated in HL [2].Clinical indications: HIV-1 infection Toxicity: torsades de pointes |
| Name | atazanavir |
|---|---|
| Synonym | More Synonyms |
| Description | Atazanavir(BMS-232632) is an highly potent HIV-1 protease inhibitor.IC50 value:Target: HIV-1 protease inhibitorAtazanavir sulfate is a sulfate salt form of atazanavir that is an highly potent HIV-1 protease inhibitor. It has a pharmacokinetic profile that supports once-daily dosing and has demonstrated a unique resistance profile and superior virologic potency compared with other antiretrovirals in vitro. In subjects with HIV, atazanavir (400 mg once daily) produced rapid and sustained improvements in viral load and CD4 counts in both antiretroviral-naive as well as previously treated patients when used in combination with dual nucleoside reverse transcriptase inhibitor (NRTI) treatment [1].After intravenous (iv), oral (po) and intraportal (ip) administration of ATV at a dosage of 7 mg/kg, AUCs in HL rats were 12.41, 5.24 and 8.89 microg/mLh, respectively, and were significantly higher than those in control rats (4.09, 1.70 and 3.38 microg/mLh). Despite the decrease of distribution volume (Vd(ss)), the terminal half-life (t(1/2)) in HL tended to be shorter than in control, and hepatic distribution of ATV in HL rats was 4.8-fold increases. These results suggested that the uptake of ATV into liver might counteract the decrease of Vd(ss). On the other hand, there was no significant difference in bioavailability, and the lymphatic transport to AUC showed no statistical change. In conclusion, although the protein binding rate and AUC were significantly increased, the pharmacokinetics of ATV might be tolerated in HL [2].Clinical indications: HIV-1 infection Toxicity: torsades de pointes |
|---|---|
| Related Catalog | |
| References |
[3]. Wood R. Atazanavir: its role in HIV treatment. Expert Rev Anti Infect Ther. 2008 Dec;6(6):785-96. [6]. Atazanavir |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Melting Point | 207-209ºC |
| Molecular Formula | C38H52N6O7 |
| Molecular Weight | 704.856 |
| Exact Mass | 704.389771 |
| PSA | 171.22000 |
| LogP | 5.20 |
| Index of Refraction | 1.562 |
| Hazard Codes | Xi |
|---|
| methyl N-[(2S)-1-[2-[(2S,3S)-2-hydroxy-3-[[(2S)-2-(methoxycarbonylamino)-3,3-dimethylbutanoyl]amino]-4-phenylbutyl]-2-[(4-pyridin-2-ylphenyl)methyl]hydrazinyl]-3,3-dimethyl-1-oxobutan-2-yl]carbamate |
| Zrivada |
| Methyl {(5S,10S,11S,14S)-11-benzyl-5-tert-butyl-10-hydroxy-15,15-dimethyl-3,6,13-trioxo-8-[4-(pyridin-2-yl)benzyl]-2-oxa-4,7,8,12-tetraazahexadecan-14-yl}carbamate |
| Atazanavir |
| BMS-232632-05 |
| 2aqu |
| [3H]-Atazanavir |
| (3S,8S,9S,12S)-3,12-Bis(1,1-dimethylethyl)-8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-((4-(2-pyridinyl)phenyl)methyl)-2,5,6,10,13-pentaazatetradecanedioic acid Dimethyl Ester |
| Methyl-{(5S,10S,11S,14S)-11-benzyl-5-tert-butyl-10-hydroxy-15,15-dimethyl-3,6,13-trioxo-8-[4-(pyridin-2-yl)benzyl]-2-oxa-4,7,8,12-tetraazahexadecan-14-yl}carbamat |
| (3S-(3R*,8R*,9R*,12R*))-3,12-Bis(1,1-dimethylethyl)-8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-((4-(2-pyridinyl)phenyl)methyl)-2,5,6,10,13-pentaazatetradecanedioic acid Dimethyl Ester |
| butanoic acid, 2-[(methoxycarbonyl)amino]-3,3-dimethyl-, 2-[(2S,3S)-2-hydroxy-3-[[(2S)-2-[(methoxycarbonyl)amino]-3,3-dimethyl-1-oxobutyl]amino]-4-phenylbutyl]-2-[[4-(2-pyridinyl)phenyl]methyl]hydrazi |
| Methyl {(5S,10S,11S,14S)-11-benzyl-10-hydroxy-15,15-dimethyl-5-(2-methyl-2-propanyl)-3,6,13-trioxo-8-[4-(2-pyridinyl)benzyl]-2-oxa-4,7,8,12-tetraazahexadecan-14-yl}carbamate |
| Reyataz |
| Butanoic acid, 2-[(methoxycarbonyl)amino]-3,3-dimethyl-, 2-[(2S,3S)-2-hydroxy-3-[[(2S)-2-[(methoxycarbonyl)amino]-3,3-dimethyl-1-oxobutyl]amino]-4-phenylbutyl]-2-[[4-(2-pyridinyl)phenyl]methyl]hydrazide, (2S)- |
| Methyl {(5S,10S,11S,14S)-11-benzyl-5-tert-butyl-10-hydroxy-15,15-dimethyl-3,6,13-trioxo-8-[4-(pyridin-2-yl)benzyl]-2-oxa-4,7,8,12-tetraazahexadecan-14-yl}carbamate (non-preferred name) |
| UNII-QZU4H47A3S |
![L-Valine, N-(methoxycarbonyl)-3-methyl-, 2-[[4-(2-pyridinyl)phenyl]methyl]hydrazide Structure](https://image.chemsrc.com/caspic/443/857904-02-0.png) CAS#:857904-02-0
CAS#:857904-02-0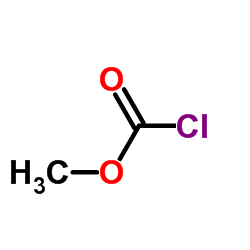 CAS#:79-22-1
CAS#:79-22-1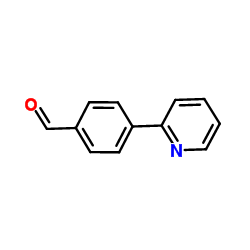 CAS#:127406-56-8
CAS#:127406-56-8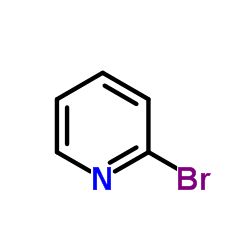 CAS#:109-04-6
CAS#:109-04-6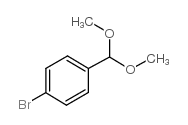 CAS#:24856-58-4
CAS#:24856-58-4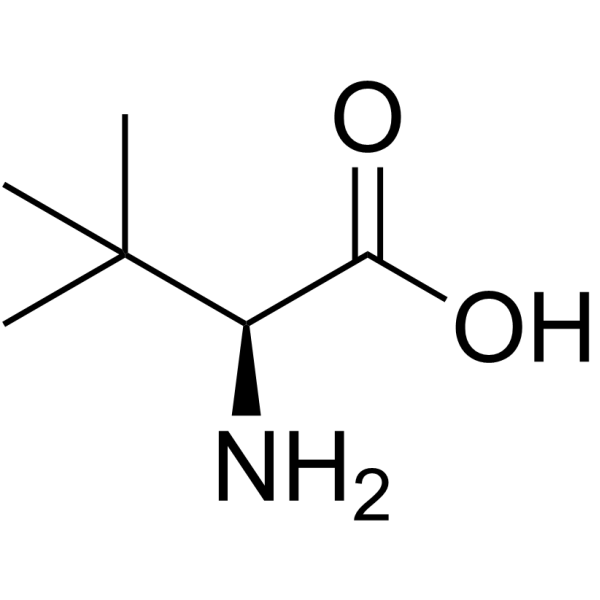 CAS#:20859-02-3
CAS#:20859-02-3![HYDRAZINECARBOXYLIC ACID, [[4-(2-PYRIDINYL)PHENYL]METHYLENE]-, 1,1-DIMETHYLETHYL ESTER Structure](https://image.chemsrc.com/caspic/274/857904-11-1.png) CAS#:857904-11-1
CAS#:857904-11-1![{(S)-1-[N'-((2S,3S)-3-amino-2-hydroxy-4-phenyl-butyl)-N'-(4-pyridin-2-yl-benzyl)-hydrazinocarbonyl]-2,2-dimethyl-propyl}-carbamic acid methyl ester Structure](https://image.chemsrc.com/caspic/216/857900-54-0.png) CAS#:857900-54-0
CAS#:857900-54-0
