etoricoxib
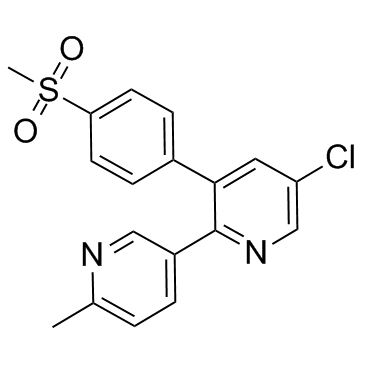
etoricoxib structure
|
Common Name | etoricoxib | ||
|---|---|---|---|---|
| CAS Number | 202409-33-4 | Molecular Weight | 358.842 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 510.0±50.0 °C at 760 mmHg | |
| Molecular Formula | C18H15ClN2O2S | Melting Point | 134-135°C | |
| MSDS | Chinese USA | Flash Point | 262.2±30.1 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of etoricoxibEtoricoxib is a non steroidal anti-inflammatory agent, acting as a selective and orally active COX-2 inhibitor, with IC50s of 1.1 μM and 116 μM for COX-2 and COX-1 in human whole blood. |
| Name | etoricoxib |
|---|---|
| Synonym | More Synonyms |
| Description | Etoricoxib is a non steroidal anti-inflammatory agent, acting as a selective and orally active COX-2 inhibitor, with IC50s of 1.1 μM and 116 μM for COX-2 and COX-1 in human whole blood. |
|---|---|
| Related Catalog | |
| Target |
COX-2:1.1 μM (IC50, in human whole blood) COX-1:116 μM (IC50, in human whole blood) |
| In Vitro | Etoricoxib (MK-0663) is a selective and orally active COX-2 inhibitor, with IC50s of 1.1 μM, 116 μM and 5 μM for COX-2, COX-1 in human whole blood and purified human COX-2, respectively. Etoricoxib shows inhibitory effect on PGE2 production by CHO (COX-2) cells (IC50, 79 nM), on purified human COX-2 with detergent (IC50, 4.1 μM), and on purified PGE2 production by U937 microsomes (low substrate; IC50, 12.1 μM). However, Etoricoxib has little activity against COX-1 with a Ki of 167 μM[1]. |
| In Vivo | Etoricoxib (0.1-30 mg/kg, p.o.) dose-dependently inhibits carrageenan-induced paw edema, carrageenan-induced paw hyperalgesia, and endotoxin-induced pyresis in rats. Etoricoxib (≥10 mg/kg) completely reverses hyperalgesia response in the rat hyperalgesia model. Etoricoxib (200 mg/kg/day) has no effect on urinary 51Cr excretion in rats, and nor in monkeys at 100 mg/kg/day[1]. Etoricoxib (50 and 100 mg/kg) potently increases the malondialdehyde (MDA) and myeloperoxidase (MPO) levels, and decreases the total glutathione (tGSH) and glutathione reductase (GSHRd) levels in rats. Etoricoxib (100 mg/kg) significantly inhibits the decrease of NO in rats[2]. Etoricoxib (0.64 mg/kg, p.o.) reduces the features such as multiple plaque lesions, hyperplasia and dysplasia induced by 1,2-dimethylhydrazine dihydrochloride (DMH) in rats[3]. |
| Animal Admin | Rats[3] Animals are assorted into the following groups with four to six animals in each group: Control Group, Animals are administrated the vehicle (1mM EDTA-saline subcutaneously) in weekly injection and 0.5% carboxymethyl cellulose per oral daily; 1,2-dimethylhydrazine dihydrochloride (DMH) Group, animals are administrated with DMH weekly at a dose of 30 mg/kg body weight subcutaneously, DMH is freshly prepared in 1mM EDTA-saline, pH adjusted to 7.0 using dilute NaOH solution; DMH + Etoricoxib Group, Etoricoxib is given daily per oral at its therapeutic anti-inflammatory dose (ED50 for rats, 0.64 mg/kg body weight) to the animals along with the weekly administration of DMH; and Etoricoxib Group: Etoricoxib alone is administered orally daily (0.64 mg/kg body weight). After six weeks, animals are kept on overnight fasting with drinking water ad libitum and sacrificed the next day. The animal body weights in all the groups are recorded once in a week till the termination.[3]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 510.0±50.0 °C at 760 mmHg |
| Melting Point | 134-135°C |
| Molecular Formula | C18H15ClN2O2S |
| Molecular Weight | 358.842 |
| Flash Point | 262.2±30.1 °C |
| Exact Mass | 358.054260 |
| PSA | 68.30000 |
| LogP | 2.21 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.601 |
| Storage condition | -20°C Freezer |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H310 |
| Precautionary Statements | P280-P302 + P350-P310 |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S26-S36 |
| RIDADR | UN 2811 6.1 / PGII |
| WGK Germany | 3 |
| HS Code | 2933399090 |
| Precursor 10 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Analgesic activity of Eugenia jambolana leave constituent: a dikaempferol rhamnopyranoside from ethyl acetate soluble fraction.
Pharm. Biol. 52(8) , 1069-78, (2014) Eugenia jambolana Lam. (Myrtaceae) is a medicinal plant used in folk medicine for the treatment of diabetes, inflammation, and pain.We investigated the antinociceptive effect of kaempferol-7-O-α-l-rha... |
|
|
Downregulation of NF-κB and PCNA in the regulatory pathways of apoptosis by cyclooxygenase-2 inhibitors in experimental lung cancer.
Mol. Cell Biochem. 369(1-2) , 75-86, (2012) Non-steroidal anti-inflammatory drugs (NSAIDs) are emerging as novel chemopreventive agents against a variety of cancers owing to their capability in blocking the tumor development by cellular prolife... |
|
|
Angiostatic role of the selective cyclooxygenase-2 inhibitor etoricoxib (MK0663) in experimental lung cancer.
Biomed. Pharmacother. 66(6) , 474-83, (2012) Lung cancer was induced in Sprague-Dawley rats by a single intra-tracheal instillation of 9,10-dimethybenz(a)anthracene (DMBA) and evaluated the anti-angiogenic action of etoricoxib, which is a select... |
| Nucoxia |
| UNII-WRX4NFY03R |
| 5-chloro-3(methylsulfonyl)phenyl-2-((4-methyl)-3-pyridyl)-pyridine |
| 5-Chloro-6'-methyl-3-(4-(methylsulfonyl)phenyl)-2,3'-bipyridine |
| Tauxib |
| Algix |
| 5-chloro-3-(4-(methylsulfonyl)phenyl)-2-(2-methyl-5-pyridinyl)pyridine |
| 5-chloro-3-(4-(methylsulfonyl)phenyl)-2-(Methyl-5-pyridinyl) pyridine |
| 5-Chloro-6'-methyl-3-[4-(methylsulfonyl)phenyl]-2,3'-bipyridine |
| etoricoxib |
| Arcoxia |
| Etoricoxibe |
| 2-(6-methylpyrid-3-yl)-3-(4-methylsulfonylphenyl)-5-chloropyridine |
| 5-chloro-2-(6-methylpyridin-3-yl)-3-(4-methylsulfonylphenyl)pyridine |
| [14C]-Etoricoxib |
| 3-(4-methylsulfonylphenyl)-2-(2-methyl-5-pyridyl)-5-chloro-pyridine |
| MK-0663 |
| 2,3'-Bipyridine, 5-chloro-6'-methyl-3-[4-(methylsulfonyl)phenyl]- |
| MK-663 |
| MFCD06797512 |
 CAS#:292067-97-1
CAS#:292067-97-1 CAS#:221615-73-2
CAS#:221615-73-2![1-(6-Methylpyridin-3-yl)-2-[4-(methylsulfonyl)phenyl]ethanone Structure](https://image.chemsrc.com/caspic/161/221615-75-4.png) CAS#:221615-75-4
CAS#:221615-75-4 CAS#:175883-59-7
CAS#:175883-59-7 CAS#:202409-86-7
CAS#:202409-86-7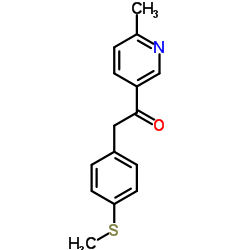 CAS#:221615-72-1
CAS#:221615-72-1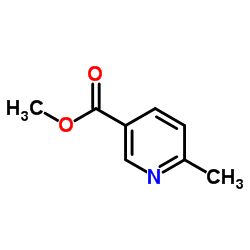 CAS#:5470-70-2
CAS#:5470-70-2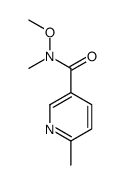 CAS#:221615-71-0
CAS#:221615-71-0 CAS#:1072-98-6
CAS#:1072-98-6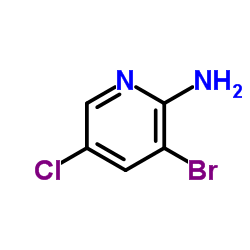 CAS#:26163-03-1
CAS#:26163-03-1 CAS#:325855-74-1
CAS#:325855-74-1