H-Trp-Phe-Tyr-Ser(PO3H2)-Pro-Arg-pNA trifluoroacetate salt
Modify Date: 2024-01-09 17:09:06
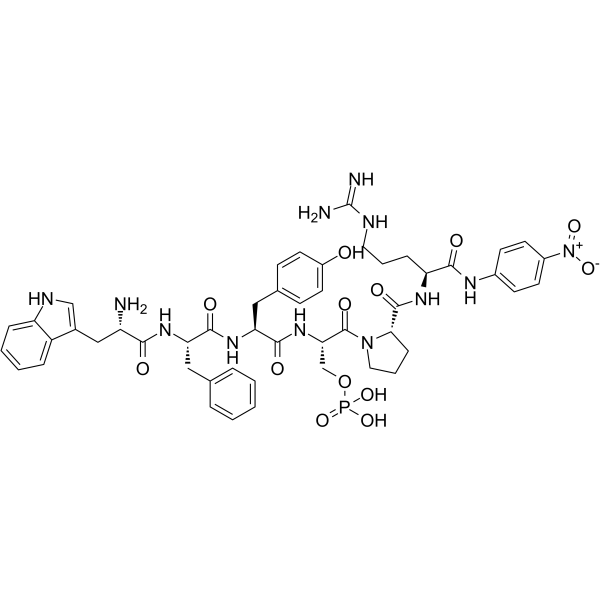
H-Trp-Phe-Tyr-Ser(PO3H2)-Pro-Arg-pNA trifluoroacetate salt structure
|
Common Name | H-Trp-Phe-Tyr-Ser(PO3H2)-Pro-Arg-pNA trifluoroacetate salt | ||
|---|---|---|---|---|
| CAS Number | 202739-41-1 | Molecular Weight | 1055.039 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C49H59N12O13P | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of H-Trp-Phe-Tyr-Ser(PO3H2)-Pro-Arg-pNA trifluoroacetate saltH-Trp-Phe-Tyr-Ser(PO3H2)-Pro-Arg-pNA is a chromogenic substrate for Pin1. Pin1 is an essential and conserved mitotic peptidyl-prolyl isomerase, and can recognize the phosphoserine-proline bonds present in mitotic phosphoproteins[1][2]. |
| Name | L-Tryptophyl-L-phenylalanyl-L-tyrosyl-O-phosphono-L-seryl-L-prolyl-N-(4-nitrophenyl)-L-argininamide |
|---|---|
| Synonym | More Synonyms |
| Description | H-Trp-Phe-Tyr-Ser(PO3H2)-Pro-Arg-pNA is a chromogenic substrate for Pin1. Pin1 is an essential and conserved mitotic peptidyl-prolyl isomerase, and can recognize the phosphoserine-proline bonds present in mitotic phosphoproteins[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Molecular Formula | C49H59N12O13P |
| Molecular Weight | 1055.039 |
| Exact Mass | 1054.406250 |
| LogP | 2.88 |
| Index of Refraction | 1.706 |
| L-Argininamide, L-tryptophyl-L-phenylalanyl-L-tyrosyl-O-phosphono-L-seryl-L-prolyl-N-(4-nitrophenyl)- |
| L-Tryptophyl-L-phenylalanyl-L-tyrosyl-O-phosphono-L-seryl-L-prolyl-N-(4-nitrophenyl)-L-argininamide |