tiliroside
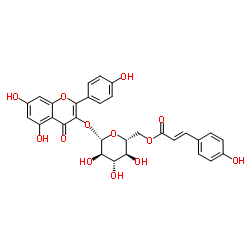
tiliroside structure
|
Common Name | tiliroside | ||
|---|---|---|---|---|
| CAS Number | 20316-62-5 | Molecular Weight | 594.520 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 943.9±65.0 °C at 760 mmHg | |
| Molecular Formula | C30H26O13 | Melting Point | 257-260ºC | |
| MSDS | Chinese USA | Flash Point | 311.9±27.8 °C | |
Use of tilirosideTiliroside, a glycosidic flavonoid, possesses anti-diabetic activities. Tiliroside is a noncompetitive inhibitor of α-amylase with a Ki value of 84.2 μM. Tiliroside inhibits carbohydrate digestion and glucose absorption in the gastrointestinal tract[1]. |
| Name | tiliroside |
|---|---|
| Synonym | More Synonyms |
| Description | Tiliroside, a glycosidic flavonoid, possesses anti-diabetic activities. Tiliroside is a noncompetitive inhibitor of α-amylase with a Ki value of 84.2 μM. Tiliroside inhibits carbohydrate digestion and glucose absorption in the gastrointestinal tract[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Tiliroside inhibits pancreatic α-amylase (IC50=0.28 mM) in vitro[1]. |
| In Vivo | In male ICR mice, the increase in postprandial plasma glucose levels was significantly suppressed in the Tiliroside-administered group[1]. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 943.9±65.0 °C at 760 mmHg |
| Melting Point | 257-260ºC |
| Molecular Formula | C30H26O13 |
| Molecular Weight | 594.520 |
| Flash Point | 311.9±27.8 °C |
| Exact Mass | 594.137329 |
| PSA | 216.58000 |
| LogP | 3.83 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.759 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| RIDADR | NONH for all modes of transport |
|---|
|
Investigation into biologically active constituents of Geum rivale L.
Acta Pol. Pharm. 70(1) , 111-4, (2013) Aerial and underground parts of Geum rivale (Rosaceae) were investigated. Tiliroside, gallic acid, ellagic acid and a sterol fraction were isolated from aerial parts of the plant. The sterol fraction ... |
|
|
New phenolic compounds with anti-adipogenic activity from the aerial parts of Pulsatilla koreana.
Planta Med. 78(16) , 1783-6, (2012) Three new phenolic compounds, pulsatillosides A (1), B (2), and C (3), were isolated from the aerial parts of Pulsatilla koreana, together with two known flavonoid glycosides, trans-tiliroside (4) and... |
|
|
Tyrosinase inhibitory effect and inhibitory mechanism of tiliroside from raspberry.
J. Enzyme Inhib. Med. Chem. 24(5) , 1154-60, (2009) Tiliroside was found to inhibit both monophenolase and diphenolase activity of mushroom tyrosinase. The lag time of tyrosine oxidation catalyzed by mushroom tyrosinase was obviously lengthened; 0.337 ... |
| 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-3-yl 6-O-[(2E)-3-(4-hydroxyphenyl)prop-2-enoyl]-b-D-glucopyranoside |
| [(2R,3S,4S,5R,6S)-6-{[5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-3-yl]oxy}-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl]methyl-(2E)-3-(4-hydroxyphenyl)acrylat |
| Kaempferol-3-Glucoside-6''-p-coumaroyl |
| tiliroside |
| [(2R,3S,4S,5R,6S)-6-{[5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-3-yl]oxy}-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl]methyl (2E)-3-(4-hydroxyphenyl)acrylate |
| 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(4-hydroxyphenyl)-3-[[6-O-[(2E)-3-(4-hydroxyphenyl)-1-oxo-2-propenyl]-β-D-glucopyranosyl]oxy]- |
| (2E)-3-(4-Hydroxyphényl)acrylate de [(2R,3S,4S,5R,6S)-6-{[5,7-dihydroxy-2-(4-hydroxyphényl)-4-oxo-4H-chromén-3-yl]oxy}-3,4,5-trihydroxytétrahydro-2H-pyran-2-yl]méthyle |
| trans-tiliroside |
| 6-O-[(2E)-3-(4-Hydroxyphényl)-2-propènan-1-oyl]-β-D-glucopyranoside de 5,7-dihydroxy-2-(4-hydroxyphényl)-4-oxo-4H-chromén-3-yle |
| 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-3-yl 6-O-[(2E)-3-(4-hydroxyphenyl)-2-propenoyl]-β-D-glucopyranoside |
| 6'-O-trans-p-CouMaroylastragalin |
| 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-3-yl 6-O-[(2E)-3-(4-hydroxyphenyl)prop-2-enoyl]-β-D-glucopyranoside |
| 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(4-hydroxyphenyl)-3-[[6-O-[(2E)-3-(4-hydroxyphenyl)-1-oxo-2-propen-1-yl]-β-D-glucopyranosyl]oxy]- |
| 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-3-yl-6-O-[(2E)-3-(4-hydroxyphenyl)-2-propenoyl]-β-D-glucopyranoside |

