2(1H)-Quinolinone,7-hydroxy-4-methyl-
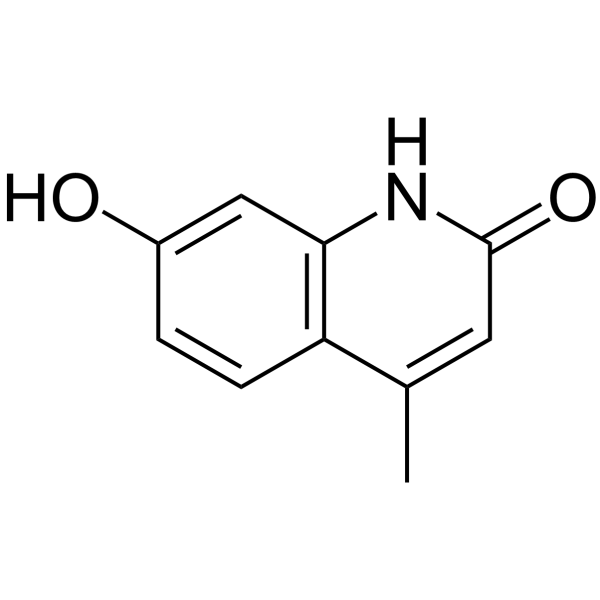
2(1H)-Quinolinone,7-hydroxy-4-methyl- structure
|
Common Name | 2(1H)-Quinolinone,7-hydroxy-4-methyl- | ||
|---|---|---|---|---|
| CAS Number | 20513-71-7 | Molecular Weight | 175.18400 | |
| Density | 1.264 g/cm3 | Boiling Point | 417.8ºC at 760 mmHg | |
| Molecular Formula | C10H9NO2 | Melting Point | ≥250ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 206.5ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 2(1H)-Quinolinone,7-hydroxy-4-methyl-7-Hydroxy-4-methyl-2(1H)-quinolone (compound 2b) is a fluorescent hydroxylated product. 7-Hydroxy-4-methyl-2(1H)-quinolone can be used for detecting hydroxyl radicals of DNA damage[1]. |
| Name | 7-Hydroxy-4-methyl-2(1H)-quinolone |
|---|---|
| Synonym | More Synonyms |
| Description | 7-Hydroxy-4-methyl-2(1H)-quinolone (compound 2b) is a fluorescent hydroxylated product. 7-Hydroxy-4-methyl-2(1H)-quinolone can be used for detecting hydroxyl radicals of DNA damage[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.264 g/cm3 |
|---|---|
| Boiling Point | 417.8ºC at 760 mmHg |
| Melting Point | ≥250ºC(lit.) |
| Molecular Formula | C10H9NO2 |
| Molecular Weight | 175.18400 |
| Flash Point | 206.5ºC |
| Exact Mass | 175.06300 |
| PSA | 53.09000 |
| LogP | 1.54210 |
| Vapour Pressure | 1.42E-07mmHg at 25°C |
| Index of Refraction | 1.611 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933790090 |
| Precursor 8 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933790090 |
|---|---|
| Summary | 2933790090. other lactams. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:9.0%. General tariff:20.0% |
|
On the metabolism of quinoline and isoquinoline: possible molecular basis for differences in biological activities.
Carcinogenesis 4 , 1169-1173, (1983) Quinoline is a hepatocarcinogen in mice and rats, a mutagen in Salmonella typhimurium, and induces unscheduled DNA synthesis in primary cultures of rat hepatocytes. In contrast, isoquinoline has not b... |
|
|
Microbial metabolism of quinoline and related compounds. XIX. Degradation of 4-methylquinoline and quinoline by Pseudomonas putida K1.
Biol. Chem. Hoppe-Seyler 374 , 479-488, (1993) A bacterial strain, designated K1, which utilizes 4-methylquinoline and quinoline as sole source of carbon, nitrogen and energy was isolated from soil. Based on its morphological and physiological cha... |
| 7-hydroxy-4-methyl-1H-quinolin-2-one |
| 2,7-Dihydroxy-4-methylquinoline 7-Hydroxy-4-methylcarbostyryl |
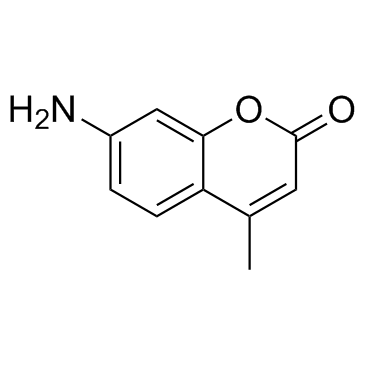
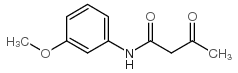
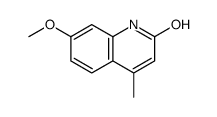
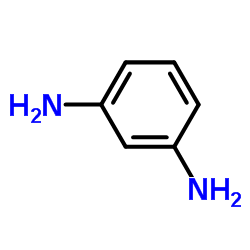
 CAS#:131195-83-0
CAS#:131195-83-0