Cefapirin
Modify Date: 2024-01-02 10:41:15

Cefapirin structure
|
Common Name | Cefapirin | ||
|---|---|---|---|---|
| CAS Number | 21593-23-7 | Molecular Weight | 423.46300 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C17H17N3O6S2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of CefapirinCephapirin (Cefapirin) is an ephalosporin antibiotic with broad-spectrum antimicrobial activity[1][2][3]. |
| Name | cephapirin |
|---|---|
| Synonym | More Synonyms |
| Description | Cephapirin (Cefapirin) is an ephalosporin antibiotic with broad-spectrum antimicrobial activity[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Cephapirin (Cefapirin) inhibits Staphylococcus aureus by cephapirin concentrations of 0.09 to 12.5 mug/mL[2]. Cephapirin (Cefapirin) inhibits S. epidermidis, S. viridans, S. pyogenes, and Diplococcus pneumonia isolates by less than 1 mug/mL[2]. |
| In Vivo | Cephapirin (Cefapirin) (200 mg; i.m.;cows) is effective for cows infected with S. aureus[3]. |
| References |
| Molecular Formula | C17H17N3O6S2 |
|---|---|
| Molecular Weight | 423.46300 |
| Exact Mass | 423.05600 |
| PSA | 179.99000 |
| LogP | 1.25360 |
| Vapour Pressure | 6.98E-26mmHg at 25°C |
| (6R,7R)-3-(acetyloxymethyl)-8-oxo-7-[(2-pyridin-4-ylsulfanylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| Cefapirin |
| Cefapirinum |
| Cefapirina |
| Cephapirine |
| 7-(2-(4-pyridylthio)acetamido)cephalosporanic acid |
| Cefapirine |
| Cefaprin sodium |
| CEPR |
| Cefaprin |
| Cefa |
| (6R)-3-acetoxymethyl-8-oxo-7t-(2-pyridin-4-ylsulfanyl-acetylamino)-(6rH)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
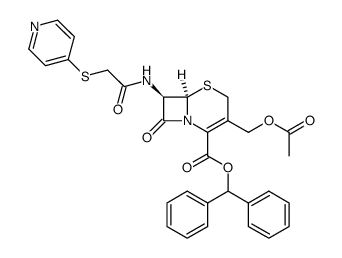 CAS#:78754-13-9
CAS#:78754-13-9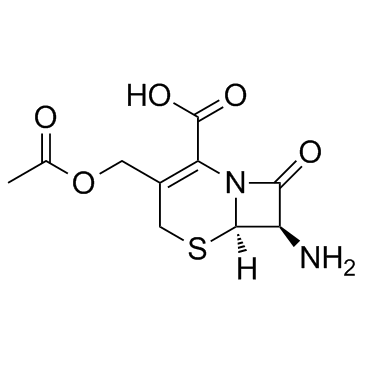 CAS#:957-68-6
CAS#:957-68-6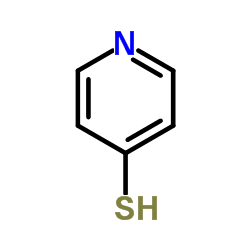 CAS#:4556-23-4
CAS#:4556-23-4![(6R,7R)-3-(acetyloxymethyl)-7-[(2-bromoacetyl)amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid Structure](https://image.chemsrc.com/caspic/359/26973-80-8.png) CAS#:26973-80-8
CAS#:26973-80-8