GSK3368715 trihydrochloride
Modify Date: 2024-01-17 15:44:14
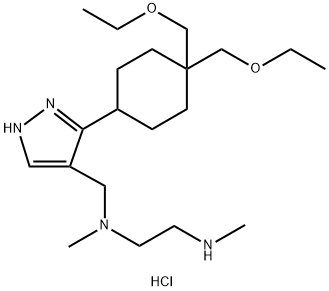
GSK3368715 trihydrochloride structure
|
Common Name | GSK3368715 trihydrochloride | ||
|---|---|---|---|---|
| CAS Number | 2227587-26-8 | Molecular Weight | 475.92 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C20H41Cl3N4O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of GSK3368715 trihydrochlorideGSK3368715 trihydrochloride (EPZ019997) is an orally active, reversible, and S-adenosyl-L-methionine (SAM) uncompetitive type I protein arginine methyltransferases (PRMTs) inhibitor (IC50=3.1 nM (PRMT1), 48 nM (PRMT3), 1148 nM (PRMT4), 5.7 nM (PRMT6), 1.7 nM (PRMT8)). GSK3368715 trihydrochloride (EPZ019997) produces a shift in arginine methylation states, alters exon usage, and has strong anti-cancer activity[1]. |
| Name | GSK3368715 3HCl |
|---|
| Description | GSK3368715 trihydrochloride (EPZ019997) is an orally active, reversible, and S-adenosyl-L-methionine (SAM) uncompetitive type I protein arginine methyltransferases (PRMTs) inhibitor (IC50=3.1 nM (PRMT1), 48 nM (PRMT3), 1148 nM (PRMT4), 5.7 nM (PRMT6), 1.7 nM (PRMT8)). GSK3368715 trihydrochloride (EPZ019997) produces a shift in arginine methylation states, alters exon usage, and has strong anti-cancer activity[1]. |
|---|---|
| Related Catalog | |
| Target |
PRMT4:1148 nM (IC50) PRMT3:48 nM (IC50) PRMT1:3.1 nM (IC50) PRMT6:5.8 nM (IC50) PRMT8:1.7 nM (IC50) |
| In Vitro | GSK3368715 trihydrochloride (EPZ019997) shows 50% or more growth inhibition relative to DMSO-treated cells in the majority of 249 cancer cell lines, representing 12 tumor types[1]. |
| In Vivo | GSK3368715 trihydrochloride (EPZ019997) significantly effects on the growth of BxPC3 xenografts at all doses tested, reducing tumor growth by 78% and 97% in the 150- and 300-mg/kg dose groups, respectively[1]. |
| References |
| Molecular Formula | C20H41Cl3N4O2 |
|---|---|
| Molecular Weight | 475.92 |
| Storage condition | -20°C |