Neferine
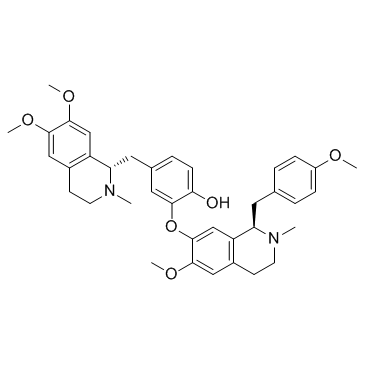
Neferine structure
|
Common Name | Neferine | ||
|---|---|---|---|---|
| CAS Number | 2292-16-2 | Molecular Weight | 624.766 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 710.9±60.0 °C at 760 mmHg | |
| Molecular Formula | C38H44N2O6 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 383.8±32.9 °C | |
Use of NeferineNeferine is a major bisbenzylisoquinline alkaloid. Neferine strongly inhibits NF-κB activation. |
| Name | 4-[[(1R)-6,7-dimethoxy-2-methyl-3,4-dihydro-1H-isoquinolin-1-yl]methyl]-2-[[(1R)-6-methoxy-1-[(4-methoxyphenyl)methyl]-2-methyl-3,4-dihydro-1H-isoquinolin-7-yl]oxy]phenol |
|---|---|
| Synonym | More Synonyms |
| Description | Neferine is a major bisbenzylisoquinline alkaloid. Neferine strongly inhibits NF-κB activation. |
|---|---|
| Related Catalog | |
| Target |
p65 Autophagy |
| In Vitro | Neferine down regulates hypoxia induced NF-κB p65 nuclear translocation and COX-2 expressions[1]. Neferine reduces high-glucose-induced collagen production and inhibits TGF-β1-Smad, ERK and p38 MAPK signaling activation in cardiac fibroblasts. Cardiac fibroblasts (CFs) are cultured in HG medium with varying concentrations of Neferine (1, 2, or 5 μM). CCK-8 assays are carried out at different time points (24, 48, and 72 h). Compared with normal glucose (NG) and osmotic control (OC) treatments, High glucose (30 mM) treatment significantly increases the proliferation of CFs in a time-dependent manner (P<0.05). High glucose (HG)-induced CF proliferation is markedly attenuated by Neferine treatment at either 2 or 5 μM compared with vehicle treatment. However, 1 μM Neferine does not inhibit HG-induced proliferation of CFs. Therefore, 2 and 5 μM Neferine are used in the remaining experiments[2]. |
| In Vivo | Neferine treatment at both low-dose (60 mg/kg/day by gavage) and high-dose (120 mg/kg/day by gavage) reduces the increment of collagen I, III and TGF-β1 protein expression induced by hyperglycemia[2]. |
| Cell Assay | Cardiac fibroblasts (CFs) are isolated from neonatal mouse ventricular tissues. After starvation in serum-free medium for 24 h, CFs are incubated in DMEM containing 5.6 mM glucose (normal glucose; NG), 30 mM D-glucose (HG), 30 mM D-glucose plus 1 μM Neferine, 30 mM D-glucose plus 2 μM Neferine, 30 mM D-glucose plus 5 μM Neferine, and 5.6 mM glucose plus 27.5 mM mannose. Cells are harvested at 24 h, 48 h, and 72h. Cell proliferation is measured with the Cell Counting Kit-8 (CCK-8) and the Cell-LightTM EdU assay[2]. |
| Animal Admin | Mice[2] Eight-week-old C57BL/6J male mice are used. Diabetes is induced by intraperitoneal injection of Streptozotocin dissolved in citrate buffer (pH 4.5) at 60 mg/kg body weight for five consecutive days. Control mice are injected with citrate buffer only. Whole blood glucose in mouse tail blood is detected with an Accu-Check Active glucometer. Mice with blood glucose concentrations higher than 18 mM are considered as diabetic animals and used in this study. The animals are randomly divided into four groups of eight animals each. Diabetic mice are divided into three groups: group 1, the diabetic control group (DM); group 2, which receive Neferine at a dose of 60 mg/kg/day (DM-NL); and group 3, which receive Neferine at a dose of 120 mg/kg/day (DM-NH). Neferine is administered twice per day by intragastric gavage for 12 weeks. Equivalent volumes of normal sodium are administered to the normal and DM control groups by gavage. Mice are anaesthetized and sacrificed at the end of the 12-week treatment[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 710.9±60.0 °C at 760 mmHg |
| Molecular Formula | C38H44N2O6 |
| Molecular Weight | 624.766 |
| Flash Point | 383.8±32.9 °C |
| Exact Mass | 624.319946 |
| PSA | 72.86000 |
| LogP | 5.49 |
| Appearance of Characters | white to beige |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C |
| Index of Refraction | 1.601 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: >15mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | C |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | SM9495000 |
|
In vitro opioid receptor affinity and in vivo behavioral studies of Nelumbo nucifera flower.
J. Ethnopharmacol. 174 , 57-65, (2015) Nelumbo nucifera Geartn., known as sacred lotus, has been used traditionally in South East Asia as a traditional medicine for various CNS disorders including stress, fever, depression, insomnia, and c... |
|
|
Anti-amnesic activity of neferine with antioxidant and anti-inflammatory capacities, as well as inhibition of ChEs and BACE1.
Life Sci. 87(13-14) , 420-30, (2010) the multifunctional potential of neferine derived from the embryo of Nelumbo nucifera seeds for the age-related neurodegenerative disorders, in vivo anti-amnesic activities and in vitro cholinesterase... |
|
|
Blockade of HERG K+ channel by isoquinoline alkaloid neferine in the stable transfected HEK293 cells.
Naunyn Schmiedebergs Arch. Pharmacol. 380(2) , 143-51, (2009) We studied the effects of isoquinoline alkaloid neferine (Nef) extracted from the seed embryo of Nelumbo nucifera Gaertn on Human ether-à-go-go-related gene (HERG) channels stably expressed in human e... |
| Neferine |
| 4-{[(1R)-6,7-Dimethoxy-2-methyl-1,2,3,4-tetrahydro-1-isoquinolinyl]methyl}-2-{[(1R)-6-methoxy-1-(4-methoxybenzyl)-2-methyl-1,2,3,4-tetrahydro-7-isoquinolinyl]oxy}phenol |
| 4-{[(1r)-6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinolin-1-yl]methyl}-2-{[(1r)-6-methoxy-1-(4-methoxybenzyl)-2-methyl-1,2,3,4-tetrahydroisoquinolin-7-yl]oxy}phenol |
| Phenol, 4-[[(1R)-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-isoquinolinyl]methyl]-2-[[(1R)-1,2,3,4-tetrahydro-6-methoxy-1-[(4-methoxyphenyl)methyl]-2-methyl-7-isoquinolinyl]oxy]- |
| Neferin |
| Phenol,4-((1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-isoquinolinyl)methyl)-2-((1,2,3,4-tetrahydro-6-methoxy-1-((4-methoxyphenyl)methyl)-2-methyl-7-isoquinolinyl)oxy) |

