S-Octylglutathione
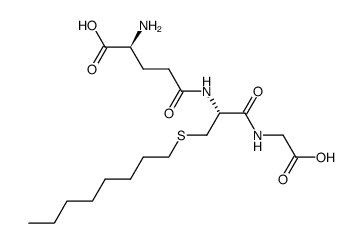
S-Octylglutathione structure
|
Common Name | S-Octylglutathione | ||
|---|---|---|---|---|
| CAS Number | 24435-27-6 | Molecular Weight | 419.53600 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C18H33N3O6S | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
Use of S-OctylglutathioneS-Octylglutathione is a competitive glutathione S-transferase (GST) inhibitor[1]. |
| Name | S-Octylglutathione |
|---|---|
| Synonym | More Synonyms |
| Description | S-Octylglutathione is a competitive glutathione S-transferase (GST) inhibitor[1]. |
|---|---|
| Related Catalog |
| Molecular Formula | C18H33N3O6S |
|---|---|
| Molecular Weight | 419.53600 |
| Exact Mass | 419.20900 |
| PSA | 184.12000 |
| LogP | 2.43990 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
|
Crystallographic and thermodynamic analysis of the binding of S-octylglutathione to the Tyr 7 to Phe mutant of glutathione S-transferase from Schistosoma japonicum.
Biochemistry 44 , 1174-1183, (2005) Glutathione S-transferases are a family of multifunctional enzymes involved in the metabolism of drugs and xenobiotics. Two tyrosine residues, Tyr 7 and Tyr 111, in the active site of the enzyme play ... |
|
|
An XAS investigation of product and inhibitor complexes of Ni-containing GlxI from Escherichia coli: mechanistic implications.
Biochemistry 40(15) , 4569-82, (2001) Escherichia coli glyoxalase I (GlxI) is a metalloisomerase that is maximally activated by Ni(2+), unlike other known GlxI enzymes which are active with Zn(2+). The metal is coordinated by two aqua lig... |
|
|
Effects of phenol compounds, glutathione analogues and a diuretic drug on glutathione S-transferase, glutathione reductase and glutathione peroxidase from canine erythrocytes.
Comp. Biochem. Physiol.,. B. 103(4) , 863-7, (1992) 1. Phenol compounds (ellagic acid, quercetin and purpurogallin), glutathione analogues (S-hexylglutathione and S-octylglutathione) and a diuretic drug (ethacrynic acid) were compared for their inhibit... |
| (S)-2-Amino-4-[(R)-1-(carboxymethyl-carbamoyl)-2-octylsulfanyl-ethylcarbamoyl]-butyric acid |