Poly(1-vinylpyrrolidone-co-vinyl acetate)
Modify Date: 2024-01-02 22:31:55
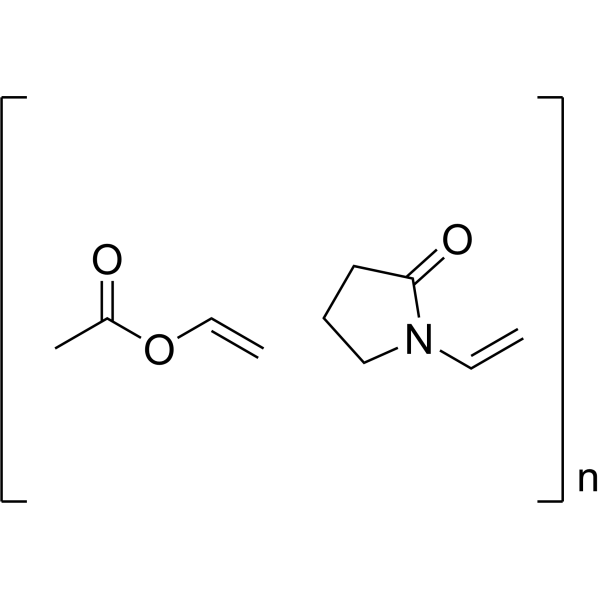
Poly(1-vinylpyrrolidone-co-vinyl acetate) structure
|
Common Name | Poly(1-vinylpyrrolidone-co-vinyl acetate) | ||
|---|---|---|---|---|
| CAS Number | 25086-89-9 | Molecular Weight | 486.726 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 536.7±50.0 °C at 760 mmHg | |
| Molecular Formula | (C6H9NO.C4H6O2)n | Melting Point | N/A | |
| MSDS | USA | Flash Point | 254.7±28.5 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Poly(1-vinylpyrrolidone-co-vinyl acetate)Copovidone can be used as an excipient, such as Film formers, adhesives, etc. Pharmaceutical excipients, or pharmaceutical auxiliaries, refer to other chemical substances used in the pharmaceutical process other than pharmaceutical ingredients. Pharmaceutical excipients generally refer to inactive ingredients in pharmaceutical preparations, which can improve the stability, solubility and processability of pharmaceutical preparations. Pharmaceutical excipients also affect the absorption, distribution, metabolism, and elimination (ADME) processes of co-administered drugs[1]. |
| Name | Poly(1-vinylpyrrolidone-co-vinyl acetate) |
|---|---|
| Synonym | More Synonyms |
| Description | Copovidone can be used as an excipient, such as Film formers, adhesives, etc. Pharmaceutical excipients, or pharmaceutical auxiliaries, refer to other chemical substances used in the pharmaceutical process other than pharmaceutical ingredients. Pharmaceutical excipients generally refer to inactive ingredients in pharmaceutical preparations, which can improve the stability, solubility and processability of pharmaceutical preparations. Pharmaceutical excipients also affect the absorption, distribution, metabolism, and elimination (ADME) processes of co-administered drugs[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 536.7±50.0 °C at 760 mmHg |
| Molecular Formula | (C6H9NO.C4H6O2)n |
| Molecular Weight | 486.726 |
| Flash Point | 254.7±28.5 °C |
| Exact Mass | 486.370911 |
| PSA | 46.61000 |
| LogP | 9.76 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.517 |
| Stability | Stable. Combustible, especially in powdered form. Incompatible with strong oxidising agents, strong reducing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xi |
| Risk Phrases | R10 |
| Safety Phrases | S22-S24/25 |
| RIDADR | UN 1987 3/PG 2 |
| WGK Germany | 1 |
| RTECS | AH3539000 |
| Packaging Group | II |
| Hazard Class | 3.0 |
| copolyvidone |
| kolima35 |
| gaf-s630 |
| Cholest-8(14)-ene-3,6-diyl diacetate |
| kolima10 |
| s630 |
| Cholest-8(14)-ene-3,6-diol, diacetate |
| MFCD00134018 |
| pvp-va |
| VP/VA Copolymers Powder |

