Iminostilbene

Iminostilbene structure
|
Common Name | Iminostilbene | ||
|---|---|---|---|---|
| CAS Number | 256-96-2 | Molecular Weight | 193.244 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 349.1±22.0 °C at 760 mmHg | |
| Molecular Formula | C14H11N | Melting Point | 197 °C | |
| MSDS | Chinese USA | Flash Point | 178.4±17.8 °C | |
| Symbol |


GHS07, GHS09 |
Signal Word | Warning | |
Use of IminostilbeneIminostilbene is a a chemical precursor of carbamazepine[1]. |
| Name | 5H-dibenzo[b,f]azepine |
|---|---|
| Synonym | More Synonyms |
| Description | Iminostilbene is a a chemical precursor of carbamazepine[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 349.1±22.0 °C at 760 mmHg |
| Melting Point | 197 °C |
| Molecular Formula | C14H11N |
| Molecular Weight | 193.244 |
| Flash Point | 178.4±17.8 °C |
| Exact Mass | 193.089142 |
| PSA | 12.03000 |
| LogP | 4.11 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.627 |
| Storage condition | Refrigerator |
| Water Solubility | dioxane: 50 mg/mL, clear |
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H411 |
| Precautionary Statements | P273 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R51/53 |
| Safety Phrases | S26-S36/37/39-S45-S61 |
| RIDADR | UN 3265 8/PG 2 |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Free-radical-scavenging effect of carbazole derivatives on AAPH-induced hemolysis of human erythrocytes.
Bioorg. Med. Chem. 15 , 1903-13, (2007) Since the research on antioxidants provides theoretical information for the medicinal development, and supplies some in vitro methods for quick-optimizing drugs, it attracts more scientific attention ... |
|
|
Simulation of the hepatic metabolism of stilbene and its tricyclic derivatives by Fenton and Ruff reagents: models for cytochrome P-450 activation of chemical carcinogens.
Biopharm. Drug Dispos. 11(1) , 39-51, (1990) Reactions of trans-stilbene, cis-stilbene, 5H-dibenzo [a,d] cyclo-heptene 5-one and 5H-dibenz [b,f] azepine (iminostilbene) with Fenton reagent [Fe (II)/H2O2] clearly simulate their hepatic metabolism... |
|
|
Synthesis and antioxidant properties of some novel 5H-dibenz[b,f]azepine derivatives in different in vitro model systems.
Eur. J. Med. Chem. 45 , 2-10, (2010) A series of 5H-dibenz[b,f]azepine containing different aminophenols and substituted aminophenols were synthesized. 3-chloro-1-(5H-dibenz[b,f]azepine-5yl)propan-1-one (2) was obtained by N-acylation of... |
| dibenz[b,f]azepine |
| MFCD00799229 |
| Minostilbene |
| EINECS 249-478-4 |
| 5H-Dibenz[b,f]azepine |
| 2,2'-Iminostilbense |
| Iminostilbene |
| Iminostilben |
| R-FMOC |
| 5H-Dibenzo[b,f]azepine |
| dibenzazepine |
| 5H-DIBENZ(B,F)AZEPINE |
![10,11-dihydro-5H-dibenzo[b,f]azepin-10-o Structure](https://image.chemsrc.com/caspic/106/4014-77-1.png) CAS#:4014-77-1
CAS#:4014-77-1 CAS#:2039-88-5
CAS#:2039-88-5 CAS#:95-51-2
CAS#:95-51-2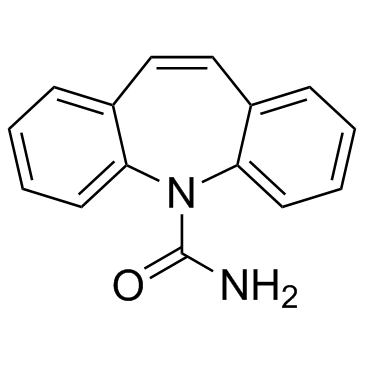 CAS#:298-46-4
CAS#:298-46-4![10-bromo-dibenz[b,f]azepine Structure](https://image.chemsrc.com/caspic/073/75272-34-3.png) CAS#:75272-34-3
CAS#:75272-34-3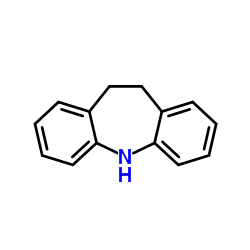 CAS#:494-19-9
CAS#:494-19-9 CAS#:108-86-1
CAS#:108-86-1 CAS#:121-46-0
CAS#:121-46-0 CAS#:615-36-1
CAS#:615-36-1 CAS#:33948-22-0
CAS#:33948-22-0 CAS#:611-64-3
CAS#:611-64-3![1-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)ethanone structure](https://image.chemsrc.com/caspic/070/13080-75-6.png) CAS#:13080-75-6
CAS#:13080-75-6 CAS#:4217-52-1
CAS#:4217-52-1![5-Cyano-5H-dibenz[b,f]azepine structure](https://image.chemsrc.com/caspic/033/42787-75-7.png) CAS#:42787-75-7
CAS#:42787-75-7 CAS#:41359-09-5
CAS#:41359-09-5![benzo[b][1]benzazepin-3-one structure](https://image.chemsrc.com/caspic/424/21186-31-2.png) CAS#:21186-31-2
CAS#:21186-31-2 CAS#:885-23-4
CAS#:885-23-4 CAS#:19579-83-0
CAS#:19579-83-0
