CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
UX9287000
-
CHEMICAL NAME :
-
Pyrrole-2-acetic acid, 1-methyl-5-p-toluoyl-
-
CAS REGISTRY NUMBER :
-
26171-23-3
-
BEILSTEIN REFERENCE NO. :
-
0485305
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
10
-
MOLECULAR FORMULA :
-
C15-H15-N-O3
-
MOLECULAR WEIGHT :
-
257.31
-
WISWESSER LINE NOTATION :
-
T5NJ A1 BVR D1& E1VQ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
293 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from stomach
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 25,1526,1975
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Rectal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
744 mg/kg
-
TOXIC EFFECTS :
-
Biochemical - Metabolism (Intermediary) - effect on inflammation or mediation of inflammation
-
REFERENCE :
-
AFTOD7 Archivos de Farmacologia y Toxicologia. (Universidad Complutense, Facultad de Medicina, Departamento Coordinado de Farmacologia, Ciudad Universitaria, Madrid 3, Spain) V.1- 1975- Volume(issue)/page/year: 5,257,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
914 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
FRPSAX Farmaco, Edizione Scientifica. (Casella Postale 227, 27100 Pavia, Italy) V.8-43 1953-88 For publisher information, see FRMCE8 Volume(issue)/page/year: 38,90,1983
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
600 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
FRPSAX Farmaco, Edizione Scientifica. (Casella Postale 227, 27100 Pavia, Italy) V.8-43 1953-88 For publisher information, see FRMCE8 Volume(issue)/page/year: 38,90,1983
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
680 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 8,45,1977
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1107 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YHTPAD Yaoxue Tongbao. Bulletin of Pharmacology. (China International Book Trading Corp., POB 2820, Beijing, Peop. Rep. China) V.13-23, 1978-88. For publisher information, see ZYZAEU. Volume(issue)/page/year: 19,460,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - species unspecified
-
DOSE/DURATION :
-
300 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IJMRAQ Indian Journal of Medical Research. (Indian Council of Medical Research, Ansari Nagar, New Delhi 110 029, India) V.1- 1913- Volume(issue)/page/year: 81,621,1985 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
200 mg/kg/4D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from stomach
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 52,454,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
1680 mg/kg/8W-I
-
TOXIC EFFECTS :
-
Brain and Coverings - other degenerative changes Liver - other changes Nutritional and Gross Metabolic - changes in metals, not otherwise specified
-
REFERENCE :
-
TOLED5 Toxicology Letters. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1977- Volume(issue)/page/year: (Suppl),245,1992 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
20600 ug/kg
-
SEX/DURATION :
-
female 21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
REFERENCE :
-
TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 30(1),25A,1984
|
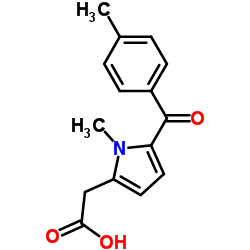
![2-[1-methyl-5-(4-methylbenzoyl)pyrrol-2-yl]acetonitrile Structure](https://image.chemsrc.com/caspic/435/26171-22-2.png) CAS#:26171-22-2
CAS#:26171-22-2 CAS#:33369-52-7
CAS#:33369-52-7 CAS#:96-54-8
CAS#:96-54-8 CAS#:21898-59-9
CAS#:21898-59-9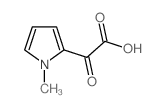 CAS#:21898-43-1
CAS#:21898-43-1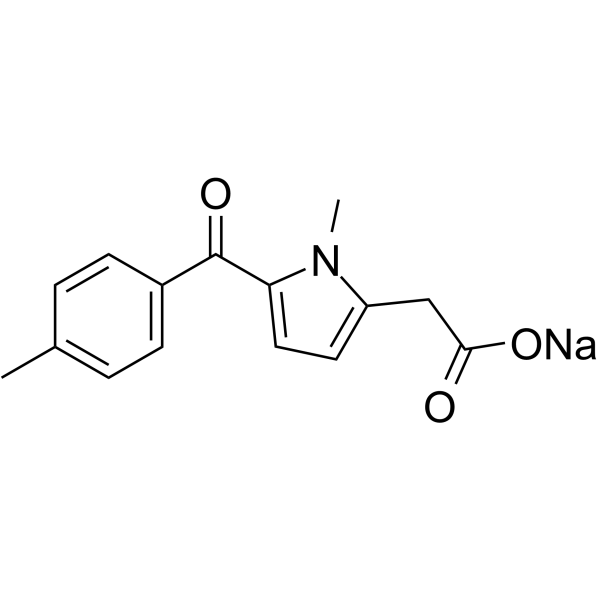 CAS#:35711-34-3
CAS#:35711-34-3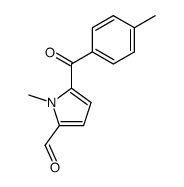 CAS#:75820-72-3
CAS#:75820-72-3![[1-methyl-5-(2-methylsulfanyl-2-methylsulfinyl-ethenyl)pyrrol-2-yl]-(4-methylphenyl)methanone Structure](https://image.chemsrc.com/caspic/310/85380-92-3.png) CAS#:85380-92-3
CAS#:85380-92-3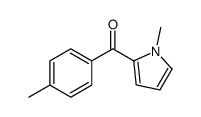 CAS#:62128-31-8
CAS#:62128-31-8 CAS#:62128-36-3
CAS#:62128-36-3![2-[3-bromo-1-methyl-5-(4-methylbenzoyl)pyrrol-2-yl]acetic acid structure](https://image.chemsrc.com/caspic/390/62380-66-9.png) CAS#:62380-66-9
CAS#:62380-66-9
