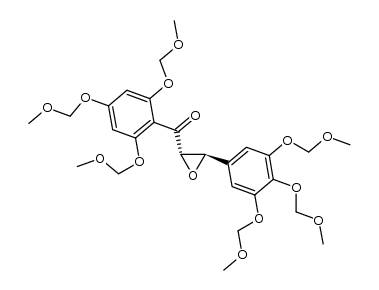
Ampelopsin
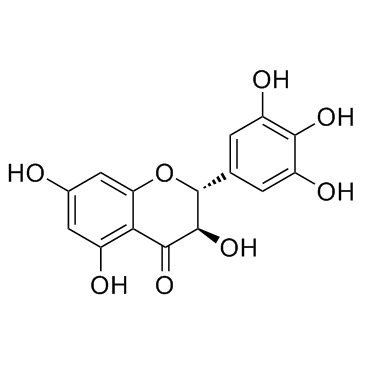
Ampelopsin structure
|
Common Name | Ampelopsin | ||
|---|---|---|---|---|
| CAS Number | 27200-12-0 | Molecular Weight | 320.251 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 780.7±60.0 °C at 760 mmHg | |
| Molecular Formula | C15H12O8 | Melting Point | 248 °C | |
| MSDS | Chinese USA | Flash Point | 296.7±26.4 °C | |
Use of AmpelopsinDihydromyricetin is a potent inhibitor with an IC50 of 48 μM on dihydropyrimidinase. Dihydromyricetin can activate autophagy through inhibiting mTOR signaling. Dihydromyricetin suppresses the formation of mTOR complexes (mTORC1/2). |
| Name | (+)-dihydromyricetin |
|---|---|
| Synonym | More Synonyms |
| Description | Dihydromyricetin is a potent inhibitor with an IC50 of 48 μM on dihydropyrimidinase. Dihydromyricetin can activate autophagy through inhibiting mTOR signaling. Dihydromyricetin suppresses the formation of mTOR complexes (mTORC1/2). |
|---|---|
| Related Catalog | |
| Target |
Dihydropyrimidinase:48 μM (IC50) mTORC1 mTORC2 Autophagy |
| In Vitro | Dihydromyricetin, a flavonol, significantly inhibits the catalytic activities of dihydropyrimidinase toward both the natural substrate dihydrouracil and xenobiotic substrate 5-propyl-hydantoin. Dihydromyricetin exhibits a significant inhibitory effect on the activities of dihydropyrimidinase for both substrates, even more than Myricetin does. The IC50 values of Dihydromyricetin for dihydropyrimidinase determined from the titration curves using Dihydrouracil and 5-propyl-hydantoin are 48±2 and 40±2 μM, respectively[1]. Dihydromyricetin (DHM) supplementation significantly reverses the increased phosphorylation of mTOR at Ser2448 (p-mTOR) during D-gal administration, which suggests that Dihydromyricetin can activate autophagy through inhibiting mTOR signaling[2]. |
| In Vivo | Changes in learning and memory capacity in rats administrated normal control group, D-gal group, D-gal+Dihydromyricetin (100 mg/kg) group, D-gal+Dihydromyricetin (200 mg/kg) group assessed by morris water maze (MWM) (n=10 per group). Dihydromyricetin (DHM) treatment significantly shortens the escape latency when compared with D-gal-induced model group[2]. |
| Kinase Assay | A rapid spectrophotometric assay is used to determine the enzymatic activity for hydantoinase, allantoinase, dihydroorotase, and imidase. Dihydrouracil, 5-propyl-hydantoin, and phthalimide are used as substrates. Unless explicitly stated otherwise, Dihydrouracil (2 mM) is used as the substrate in the standard assay of dihydropyrimidinase. Briefly, the decrease in absorbancy at 230, 248, and 298 nm is measured upon hydrolysis of Dihydrouracil, 5-propyl-hydantoin, and Phthalimide as the substrate at 25°C, respectively. To start the reaction, the purified dihydropyrimidinase (10-70 μg) is added to a 2 mL solution containing the substrate and 100 mM Tris-HCl (pH 8.0). Substrate hydrolysis is monitored with a UV/vis spectrophotometer. The extinction coefficient of each substrate is determined experimentally by direct measurement with a spectrophotometer. The extinction coefficients of Dihydrouracil, 5-propyl-hydantoin, and Phthalimide are 0.683 mM-1cm-1 at 230 nm, 0.0538 mM-1cm-1 at 248 nm, and 3.12 mM-1cm-1 at 298 nm, respectively. The initial rates of change are a function of enzyme concentration within the absorbance range of 0.01-0.18 min-1. A unit of activity is defined as the amount of enzyme catalyzing the hydrolysis of 1 μmol substrate/min, and the specific activity is expressed in terms of units of activity per milligram of enzyme. The kinetic parameters Km and Vmax are determined from a non-linear plot by fitting the hydrolyzing rate from individual experiments to the Michaelis-Menten equation[1]. |
| Cell Assay | Hippocampus and cortex tissue samples are homogenized in lysis buffer containing 20 mM Tris (pH 7.5), 135 mM NaCl, 2 mM EDTA, 2 mM DTT, 25 mM β-glycerophosphate, 2 mM sodium pyrophosphate, 10% glycerol, 1% Triton X-100, 1 mM sodium orthovanadate, 10 mM NaF, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mM PMSF for 30 min on ice and centrifuged at 12000×g at 4°C for 30 min. The supernatant is collected and protein quantification is carried out using a BCA kit. The protein samples are boiled in the presence of sample buffer at 95°C for 5 min. The target protein is separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membrane, and then probed by corresponding primary and secondary antibodies. Finally, the target protein is visualized by enhanced chemiluminescence (ECL) reagent exposure to X-ray film[2]. |
| Animal Admin | Rats[2] Totally 40 male Sprague-Dawley (SD) rats (age: 8 weeks old; body weight: 160±20 g) are used. The rats are randomly divided into four groups including normal control group, D-gal model group, and D-gal combined with DHM at the doses of 100 and 200 mg/kg-d groups with 10 rats in each group. All rats are housed at the environment with room temperature of 22±2°C and a dark-light cycle (12 h: 12h), and provided the accessibility to food and water ad libitum. After adapting to new environment for 1 week, the rats from DHM groups are administered with DHM dissolved in distilled water at the designated dosages by gavage once a day at 8:00am for 6 consecutive weeks. The rats from the normal control group are administrated with distilled water. Except from the normal control group, the rats from other groups are subjected to subcutaneous injection of D-gal at the dose of 150 mg/kg.d for 6 consecutive weeks. Each administration of DHM should be 2 h ahead of D-gal injection. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 780.7±60.0 °C at 760 mmHg |
| Melting Point | 248 °C |
| Molecular Formula | C15H12O8 |
| Molecular Weight | 320.251 |
| Flash Point | 296.7±26.4 °C |
| Exact Mass | 320.053223 |
| PSA | 147.68000 |
| LogP | 1.23 |
| Appearance of Characters | white to beige |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Index of Refraction | 1.798 |
| Storage condition | ?20°C |
| Water Solubility | DMSO: ≥5mg/mL (warmed) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~71% 
Ampelopsin CAS#:27200-12-0 |
| Literature: Li, Shaoshun; Onda, Masayuki; Kagawa, Hitoshi; Kawase, Hiromi; Iguchi, Mieko; Harigaya, Yoshihiro Journal of Heterocyclic Chemistry, 1990 , vol. 27, # 7 p. 2029 - 2035 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Sex-specific urinary biomarkers for diagnosing bipolar disorder.
PLoS ONE 9(12) , e115221, (2014) Sex-based differences are prominent in affective disorders, but there are no biomarkers available to support sex-specific, laboratory-based diagnostics for male and female bipolar disorder (BD) patien... |
|
|
Biosynthetic origin of E-resveratrol accumulation in grape canes during postharvest storage.
J. Agric. Food Chem. 63(5) , 1631-8, (2015) Grape canes are vineyard waste products containing valuable phytochemicals of medicine and agriculture interest. Grape canes storage is critical for the accumulation of these bioactive compounds. In t... |
|
|
Dihydromyricetin activates AMP-activated protein kinase and P38(MAPK) exerting antitumor potential in osteosarcoma.
Cancer Prev. Res. (Phila.) 7(9) , 927-38, (2014) Numerous patients with osteosarcoma either are not sensitive to chemotherapy or develop drug resistance to current chemotherapy regimens. Therefore, it is necessary to develop several potentially usef... |
| 4H-1-Benzopyran-4-one, 2,3-dihydro-3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-, (2S,3S)- |
| Ampelopsin E |
| (2R,3R)-3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-2,3-dihydrochromen-4-one |
| 3,5,7,3',4',5'-hexahydroxy-2,3-dihydroflavone |
| (2R,3R)-dihydromyricetin |
| Dihydromyricetin |
| (2R,3R)-3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl)-2,3-dihydrochromen-4-one Ampelopsin DHM |
| Flavanone,3,3',4',5,5',7-hexahydroxy |
| 3,5,7,3',4',5'-hexahydroxy-2,3-dihydroflavonol |
| (+)-Dihydromyricetin |
| (2S,3S)-3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl)-2,3-dihydro-4H-chromen-4-one |
| Ampelopsin |
| Ampeloptin |
| (+)-Ampelopsin |
| 3,3',4',5,5',7-Hexahydroxy-2,3-dihydroflavanonol |
| rac-ampelopsin |