S-Bioallethrin
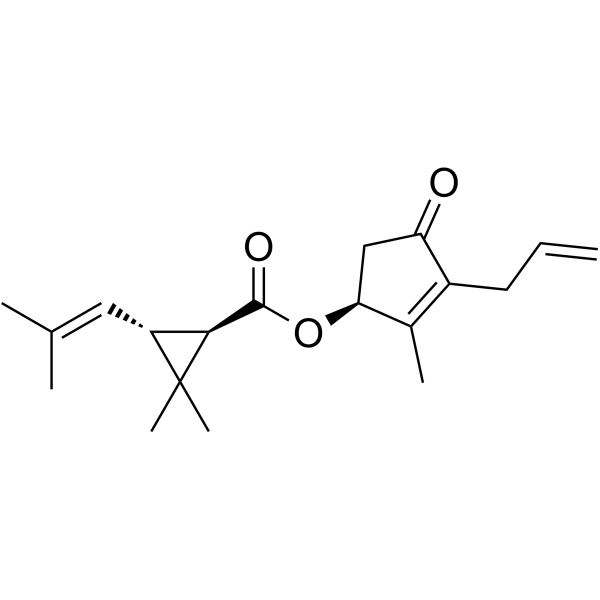
S-Bioallethrin structure
|
Common Name | S-Bioallethrin | ||
|---|---|---|---|---|
| CAS Number | 28434-00-6 | Molecular Weight | 302.40800 | |
| Density | 1.05 g/cm3 | Boiling Point | 386.8ºC at 760 mmHg | |
| Molecular Formula | C19H26O3 | Melting Point | 49.5ºC | |
| MSDS | Chinese USA | Flash Point | 166ºC | |
| Symbol |


GHS06, GHS09 |
Signal Word | Danger | |
Use of S-BioallethrinS-Bioallethrin is a pyrethroid insecticide. S-Bioallethrin disrupts nerve function by modifying the gating kinetics of transitions between the conducting and nonconducting states of voltage-gated sodium channels[1]. |
| Name | (+)-trans-(S)-allethrin |
|---|---|
| Synonym | More Synonyms |
| Description | S-Bioallethrin is a pyrethroid insecticide. S-Bioallethrin disrupts nerve function by modifying the gating kinetics of transitions between the conducting and nonconducting states of voltage-gated sodium channels[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.05 g/cm3 |
|---|---|
| Boiling Point | 386.8ºC at 760 mmHg |
| Melting Point | 49.5ºC |
| Molecular Formula | C19H26O3 |
| Molecular Weight | 302.40800 |
| Flash Point | 166ºC |
| Exact Mass | 302.18800 |
| PSA | 43.37000 |
| LogP | 4.00200 |
| Vapour Pressure | 3.46E-06mmHg at 25°C |
| Index of Refraction | 1.505 (25ºC) |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS06, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302 + H312-H330-H410 |
| Precautionary Statements | P260-P280-P301 + P312 + P330-P304 + P340 + P310-P403 + P233 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xn: Harmful;N: Dangerous for the environment; |
| Risk Phrases | R20/22 |
| Safety Phrases | 36-60-61 |
| RIDADR | UN3082 9/PG 3 |
| RTECS | GZ1472000 |
| Packaging Group | I; II; III |
| Hazard Class | 6.1 |
| HS Code | 2918300016 |
| HS Code | 2918300016 |
|---|
|
Influence of pyrethroids and piperonyl butoxide on histamine release from isolated rat mast cells.
Inflamm. Res. 56(11) , 473-8, (2007) Pyrethroids are insecticides with low acute toxicity in mammals but their world-wide use for domestic and occupational purposes has caused concern about the risks of long-term exposure. The mammalian ... |
|
|
The effects of combined exposure to the pyrethroids deltamethrin and S-bioallethrin on hippocampal inhibition and skeletal muscle hyperexcitability in rats.
Toxicol. Appl. Pharmacol. 216(2) , 354-62, (2006) The default assumption that different pyrethroid insecticides, sharing a common mode of action, will show additivity of toxicity has not always been supported by in vitro measures, some of which have ... |
|
|
Differential state-dependent modification of inactivation-deficient Nav1.6 sodium channels by the pyrethroid insecticides S-bioallethrin, tefluthrin and deltamethrin
Neurotoxicology 33(3) , 384-90, (2012) Highlights ► We constructed Nav1.6Q3 sodium channels containing the IFM/QQQ mutation. ► Nav1.6Q3 channels expressed in Xenopus oocytes exhibited persistent activation. ► Opening of Nav1.6Q3 channels e... |
| (S)-3-allyl-2-methyl-4-oxocyclopent-2-enyl (1R,3R)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate |
| (1S)-2-methyl-4-oxo-3-(2-propen-1-yl)-2-cyclopenten-1-yl (1R,3R)-2,2-dimethyl-3-(2-methyl-1-propen-1-yl)cyclopropanecarboxylate |
| S-bioallethrin |
| EINECS 249-013-5 |
| (1S)-2-methyl-4-oxo-3-(prop-2-en-1-yl)cyclopent-2-en-1-yl (1R,3R)-2,2-dimethyl-3-(2-methylprop-1-en-1-yl)cyclopropane-1-carboxylate |
| (S)-3-allyl-2-methyl-4-oxocyclopent-2-enyl (1R)-trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate |
| esdépalléthrine |
| esdepallethrine |

