AVN-944
Modify Date: 2024-01-06 14:55:03
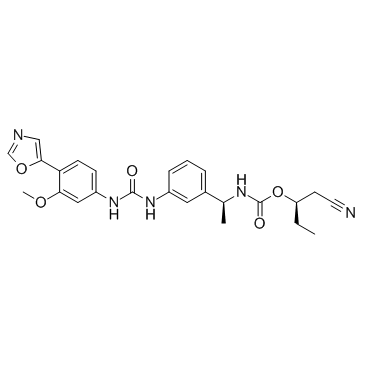
AVN-944 structure
|
Common Name | AVN-944 | ||
|---|---|---|---|---|
| CAS Number | 297730-17-7 | Molecular Weight | 477.51200 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C25H27N5O5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of AVN-944AVN-944(VX-944) is a selective, noncompetitive inhibitor of the enzyme directed against human IMPDH with Ki of 6-10 nM for IMPDH1/IMPDH2.IC50 value: 6-10 nM (Ki) [1]Target: IMPDHin vitro: AVN-944 strikingly inhibit RNA synthesis within 2 h of exposure. Depletion of guanine nucleotides by MPA and AVN-944 also causes an early and near-complete reduction in levels of the 45S precursor rRNA synthesis and the concomitant translocation of nucleolar proteins including nucleolin, nucleophosmin, and nucleostemin from the nucleolus to the nucleoplasm [2]. AVN944 induced caspase-dependentand caspase-independent cell death in LNCaP, CWR22Rv1, and DU145 cells. AVN944 induced expression of p53-target proteins Bok, Bax and Noxa in androgen-responsive cell lines and suppressed expression of survivin in prostate cancer cells regardless of their androgen sensitivity. AVN944 also induced differentiation of androgen-independent prostate cancer cells as indicated by morphological changes and increased expression of genes coding for prostasomal proteins, keratins and other proteins, including tumor suppressor genes MIG-6 and NDRG1. AVN944-differentiated androgen-independent DU145 and PC-3 cells are sensitized to TRAIL-induced apoptosis as demonstrated by induction of caspases and PARP cleavage [3]. |
| Name | [(2R)-1-cyanobutan-2-yl] N-[(1S)-1-[3-[[3-methoxy-4-(1,3-oxazol-5-yl)phenyl]carbamoylamino]phenyl]ethyl]carbamate |
|---|---|
| Synonym | More Synonyms |
| Description | AVN-944(VX-944) is a selective, noncompetitive inhibitor of the enzyme directed against human IMPDH with Ki of 6-10 nM for IMPDH1/IMPDH2.IC50 value: 6-10 nM (Ki) [1]Target: IMPDHin vitro: AVN-944 strikingly inhibit RNA synthesis within 2 h of exposure. Depletion of guanine nucleotides by MPA and AVN-944 also causes an early and near-complete reduction in levels of the 45S precursor rRNA synthesis and the concomitant translocation of nucleolar proteins including nucleolin, nucleophosmin, and nucleostemin from the nucleolus to the nucleoplasm [2]. AVN944 induced caspase-dependentand caspase-independent cell death in LNCaP, CWR22Rv1, and DU145 cells. AVN944 induced expression of p53-target proteins Bok, Bax and Noxa in androgen-responsive cell lines and suppressed expression of survivin in prostate cancer cells regardless of their androgen sensitivity. AVN944 also induced differentiation of androgen-independent prostate cancer cells as indicated by morphological changes and increased expression of genes coding for prostasomal proteins, keratins and other proteins, including tumor suppressor genes MIG-6 and NDRG1. AVN944-differentiated androgen-independent DU145 and PC-3 cells are sensitized to TRAIL-induced apoptosis as demonstrated by induction of caspases and PARP cleavage [3]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C25H27N5O5 |
|---|---|
| Molecular Weight | 477.51200 |
| Exact Mass | 477.20100 |
| PSA | 145.49000 |
| LogP | 5.76468 |
| Storage condition | 2-8℃ |
| AVN944 |
| Carbamic acid,((1S)-1-(3-((((3-methoxy-4-(5-oxazolyl)phenyl)amino)carbonyl)amino)phenyl)ethyl)-,(1R)-1-(cyanomethyl)propyl ester |
| UNII-I3NPL1V48Q |
| AVN 944 |
| AVN-944 |