Etofenamate
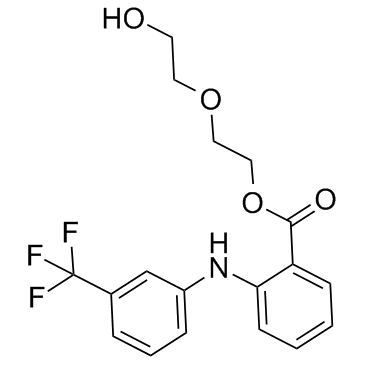
Etofenamate structure
|
Common Name | Etofenamate | ||
|---|---|---|---|---|
| CAS Number | 30544-47-9 | Molecular Weight | 369.335 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 451.1±45.0 °C at 760 mmHg | |
| Molecular Formula | C18H18F3NO4 | Melting Point | 25°C | |
| MSDS | N/A | Flash Point | 226.6±28.7 °C | |
Use of EtofenamateEtofenamate is a non-steroidal anti-inflammatory drug used for the treatment joint and muscular pain. |
| Name | Etofenamate |
|---|---|
| Synonym | More Synonyms |
| Description | Etofenamate is a non-steroidal anti-inflammatory drug used for the treatment joint and muscular pain. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 451.1±45.0 °C at 760 mmHg |
| Melting Point | 25°C |
| Molecular Formula | C18H18F3NO4 |
| Molecular Weight | 369.335 |
| Flash Point | 226.6±28.7 °C |
| Exact Mass | 369.118805 |
| PSA | 67.79000 |
| LogP | 4.14 |
| Appearance of Characters | light yellow oil |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.552 |
| Storage condition | Refrigerator |
| Water Solubility | Practically insoluble in water, miscible with ethanol (96 per cent) and with ethyl acetate. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2922509090 |
|---|
|
~% 
Etofenamate CAS#:30544-47-9 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 27, # 6 b p. 1300 - 1312 |
|
~% 
Etofenamate CAS#:30544-47-9 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 27, # 6 b p. 1300 - 1312 |
| HS Code | 2922509090 |
|---|---|
| Summary | 2922509090. other amino-alcohol-phenols, amino-acid-phenols and other amino-compounds with oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Adverse reaction of topical etofenamate: petechial eruption.
West Indian Med. J. 61(7) , 767-9, (2012) Etofenamate is a non-steroidal anti-inflammatory drug (NSAID). Clinical findings caused by etofenamate are uncommon. Allergic contact dermatitis is the most common cutaneous reaction reported. But pet... |
|
|
Turpentine sensitization in a nonsteroidal anti-inflammatory solution user.
Dermatitis 23(4) , 182-3, (2012)
|
|
|
Assessing the removal of pharmaceuticals and personal care products in a full-scale activated sludge plant.
Environ. Sci. Pollut. Res. Int. 19(5) , 1818-27, (2012) This study aimed to investigate the removal mechanisms of pharmaceutical active compounds (PhACs) and musks in a wastewater treatment plant (WWTP). Biological removal and adsorption in the activated s... |
| Benzoic acid, 2-[[3-(trifluoromethyl)phenyl]amino]-, 2-(2-hydroxyethoxy)ethyl ester |
| 2-(2-Hydroxyethoxy)ethyl fufenamate |
| 2-[[3-(Trifluoromethyl)phenyl]amino]benzoic Acid 2-(2-Hydroxyethoxy)ethyl Ester |
| Activon |
| 2-(2-hydroxyethoxy)ethyl 2-[3-(trifluoromethyl)anilino]benzoate |
| Bayrogel |
| N-(a,a,a-Trifluoro-m-tolyl)anthranilic Acid 2-(2-Hydroxyethoxy)ethyl Ester |
| UNII-KZF0XM66JC |
| 2-(2-Hydroxyethoxy)ethyl 2-{[3-(trifluoromethyl)phenyl]amino}benzoate |
| Etofenamate |
| Glasel |
| Rheumon |
| Rheumon gel |
| Bayagel |