Isobavachin
Modify Date: 2024-01-02 19:23:42
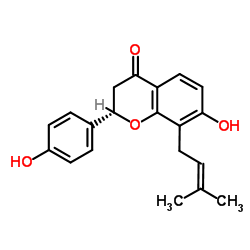
Isobavachin structure
|
Common Name | Isobavachin | ||
|---|---|---|---|---|
| CAS Number | 31524-62-6 | Molecular Weight | 324.370 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 558.3±50.0 °C at 760 mmHg | |
| Molecular Formula | C20H20O4 | Melting Point | 187-188℃ | |
| MSDS | N/A | Flash Point | 202.1±23.6 °C | |
Use of IsobavachinIsobavachin, an antioxidant isaolated from Psoralea morisiana with a prenyl group at position 8 of ring A, promotes neuronal differentiation and the potential role of its protein prenylation[1][2]. |
| Name | Isobavachin |
|---|---|
| Synonym | More Synonyms |
| Description | Isobavachin, an antioxidant isaolated from Psoralea morisiana with a prenyl group at position 8 of ring A, promotes neuronal differentiation and the potential role of its protein prenylation[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 558.3±50.0 °C at 760 mmHg |
| Melting Point | 187-188℃ |
| Molecular Formula | C20H20O4 |
| Molecular Weight | 324.370 |
| Flash Point | 202.1±23.6 °C |
| Exact Mass | 324.136169 |
| PSA | 66.76000 |
| LogP | 4.85 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.622 |
| Storage condition | 2-8C |
| HS Code | 2932999099 |
|---|
|
~46% 
Isobavachin CAS#:31524-62-6 |
| Literature: Advanced Synthesis and Catalysis, , vol. 355, # 9 p. 1817 - 1828 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| 4',7-dihydroxy-8-prenylflavanone |
| (+-)-6-chloro-7 |
| 4H-1-Benzopyran-4-one, 2,3-dihydro-7-hydroxy-2-(4-hydroxyphenyl)-8-(3-methyl-2-buten-1-yl)-, (2S)- |
| C16H16ClNO2 |
| SK&6-Chlor-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepin |
| (2S)-7-Hydroxy-2-(4-hydroxyphenyl)-8-(3-methyl-2-buten-1-yl)-2,3-dihydro-4H-chromen-4-one |
| 6-chloro-1-phenyl-2,3,4,5-tetrahydro-1h-3-benzazepine-7,8-diol |
| 6-Chloro-2,3,4,5-tetrahydro-1-phenyl-1H-3-benzazepine-7,8-diol |
| 8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine |
| 1H-3-Benzazepine-7,8-diol,6-chloro-2,3,4,5-tetrahydro-1-phenyl |
| 8-prenylliquiritigenin |
| 8-dimethylallylliquiritigenin |
| 6-chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine |