Tildipirosin
Modify Date: 2024-01-02 14:15:12
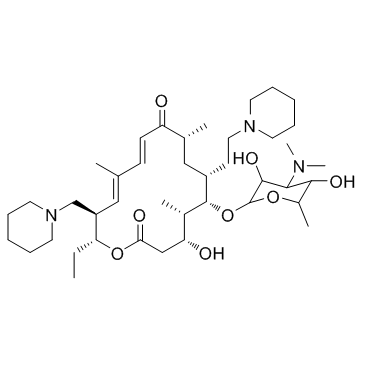
Tildipirosin structure
|
Common Name | Tildipirosin | ||
|---|---|---|---|---|
| CAS Number | 328898-40-4 | Molecular Weight | 734.018 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 846.8±65.0 °C at 760 mmHg | |
| Molecular Formula | C41H71N3O8 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 465.9±34.3 °C | |
Use of TildipirosinTildipirosin, a long-acting macrolide, has antibiotic activity. |
| Name | Tildipirosin |
|---|---|
| Synonym | More Synonyms |
| Description | Tildipirosin, a long-acting macrolide, has antibiotic activity. |
|---|---|
| Related Catalog | |
| In Vitro | Tildipirosin exhibits the inhibitory effect on C. coli species, and 23 of 31 (74%) isolates have MICs of 8 or 16 μg/mL while 8 of 31 (26%) have MIC >256 μg/mL. MICs against C. jejuni are 8-64 μg/mL. Tildipirosin against S. enterica and E. coli are 2-8 μg/mL[1]. Tildipirosin inhibits the treponeme isolates form from CODD lesions from 19 sheep, with MIC90 of 0.0469 mg/L[3]. The P. multocida B130 clones show the MIC of 0.25 mg/L for tildipirosin. The 10 P. multocida isolates that carry only erm(42) exhibit MIC of 16-32 mg/L for tildipirosin. The single M. haemolytica that harbours only erm(42) shows MIC of 32 mg/L for tildipirosin[4]. |
| In Vivo | The mean percentage of lung consolidation for tildipirosin (4 mg/kg, s.c.)-treated calves is significantly lower than those for tulathromycin-treated and control calves. Metaphylactic administration of tildipirosin to calves 5 days prior to H somni challenge prevents subsequent culture of the pathogen from bronchial secretions and is more effective in minimizing clinical disease and lung lesions than is metaphylactic administration of tulathromycin[2]. |
| Animal Admin | On day 0, each pen of 4 calves is randomly assigned by means of drawing numbers from a hat to receive 1 of 3 treatments; thus, each treatment group consists of 8 calves. Calves in group 1 receive tildipirosinb (4 mg/kg, SC), calves in group 2 receive tulathromycinc (2.5 mg/kg, SC), and calves in group 3 receive saline (0.9% NaCl) solution (1 mL/45 kg, SC; control). The volume of saline solution administered to the calves in group 3 approximates the volume of the assigned antimicrobial administered to the calves of groups 1 and 2.On day 5, all calves are experimentally inoculated (challenged) with 10 mL of PBS solution supplemented with 5% bovine fetal serum containing 1.6×109 CFUs of H somni/mL instilled via a flexible bronchoalveolar lavage tube (length, 3 m; external diameter, 11 mm; internal diameter, 3 mm) that is passed through the nasal passage and nasopharynx to the level of the tracheal bifurcation. Proper placement of the tube at the tracheal bifurcation is verified on the basis of qualitative observations that includ an elicited cough, absence of evidence of esophageal or ruminal placement as determined by smell and lack of tension and failure to observe the tube within the esophagus during placement, the presence of resistance at the carina, and the passage of the tube to a predetermined mark that approximates the distance from the nares to the carina. Following experimental inoculation, the tube is flushed with 60 mL of saline solution and 120 mL of air before it is removed from the calf.On day 8, all calves are weighed, sedated with xylazined (0.25 mg/kg), and transported in a trailer in groups of 4 to 6 calves. Immediately after euthanasia, a necropsy is performed on each calf. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 846.8±65.0 °C at 760 mmHg |
| Molecular Formula | C41H71N3O8 |
| Molecular Weight | 734.018 |
| Flash Point | 465.9±34.3 °C |
| Exact Mass | 733.524109 |
| PSA | 132.24000 |
| LogP | 4.70 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.557 |
| Storage condition | 2-8℃ |
|
~% 
Tildipirosin CAS#:328898-40-4 |
| Literature: WO2008/12343 A2, ; Page/Page column 54-55 ; |
| Oxacyclohexadeca-11,13-diene-2,10-dione, 6-[[3,6-dideoxy-3-(dimethylamino)-β-D-glucopyranosyl]oxy]-16-ethyl-4-hydroxy-5,9,13-trimethyl-7-[2-(1-piperidinyl)ethyl]-15-(1-piperidinylmethyl)-, (4R,5S,6 S,7R,9R,11E,13E,15R,16R)- |
| 20,23-dipiperidinyl-5-O-mycaminosyl-tylonolide |
| UNII-S795AT66JB |
| Zuprevo |
| Tildipirosin |
| (4R,5S,6S,7R,9R,11E,13E,15R,16R)-16-Ethyl-4-hydroxy-5,9,13-trimethyl-2,10-dioxo-7-[2-(1-piperidinyl)ethyl]-15-(1-piperidinylmethyl)oxacyclohexadeca-11,13-dien-6-yl 3,6-dideoxy-3-(dimethylamino)-β-D -glucopyranoside |