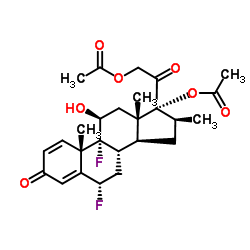
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
TU3722000
-
CHEMICAL NAME :
-
Pregna-1,4-diene-3,20-dione, 17,21-bis(acetyloxy)-6,9-difluoro-11-hydroxy-16-methy l-, (6-alpha,11-beta,16-beta)-
-
CAS REGISTRY NUMBER :
-
33564-31-7
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
14
-
MOLECULAR FORMULA :
-
C26-H32-F2-O7
-
MOLECULAR WEIGHT :
-
494.58
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: -,487,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: -,487,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: -,487,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: -,487,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: -,487,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: -,487,1990 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3500 ug/kg/5W-C
-
TOXIC EFFECTS :
-
Endocrine - changes in thymus weight Blood - changes in leukocyte (WBC) count Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 27,1217,1984 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
4950 ug/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Newborn - stillbirth Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 28,207,1984
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
495 ug/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 28,207,1984
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
225 ug/kg
-
SEX/DURATION :
-
female 17-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 28,225,1984
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
4500 ng/kg
-
SEX/DURATION :
-
female 17-22 day(s) after conception lactating female 4 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 28,225,1984
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
208 ug/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - body wall
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 28,241,1984
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
208 ug/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Effects on Newborn - live birth index (measured after birth)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 28,241,1984
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
3250 ng/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 28,241,1984
|