Celastrol
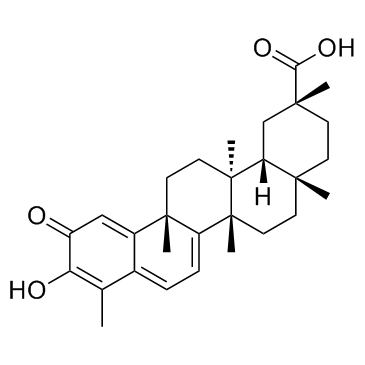
Celastrol structure
|
Common Name | Celastrol | ||
|---|---|---|---|---|
| CAS Number | 34157-83-0 | Molecular Weight | 450.610 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 645.7±55.0 °C at 760 mmHg | |
| Molecular Formula | C29H38O4 | Melting Point | 185-200ºC | |
| MSDS | Chinese USA | Flash Point | 358.3±28.0 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of CelastrolTripterin (Celastrol) is a proteasome inhibitor which potently and preferentially inhibits the chymotrypsin-like activity of a purified 20S proteasome with IC50 of 2.5 μM. |
| Name | celastrol |
|---|---|
| Synonym | More Synonyms |
| Description | Tripterin (Celastrol) is a proteasome inhibitor which potently and preferentially inhibits the chymotrypsin-like activity of a purified 20S proteasome with IC50 of 2.5 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 2.5 μM (20S proteasome)[1] |
| In Vitro | Tripterin (Celastrol) significantly inhibits the proteasomal chymotrypsin activity in PC-3 cells in a concentration-dependent manner; at 2.5 μM it reaches ~55% inhibition, comparable to its potency to a purified 20S proteasome (IC50=2.5 μM). Furthermore, increased levels of IκB-α, Bax, and p27 are observed, three well known target proteins of the proteasome in PC-3 cells treated with Celastrol[1]. |
| In Vivo | Treatment of PC-3 tumor-bearing nude mice with Tripterin (Celastrol) (1-3 mg/kg/d, i.p., 1-31 days) results in significant inhibition (65-93%) of the tumor growth[1]. Following treatment with 3 and 6 mg/kg Tripterin (Celastrol), the levels of malondialdehyde (MDA) are significantly decreased by 35.2 and 36.7% (P<0.05), respectively. Treatment with 3 and 6 mg/kg Tripterin (Celastrol) markedly restores the GSH level (P<0.05) to almost normal levels[2]. |
| Kinase Assay | A purified rabbit 20S proteasome (0.1 μg) is incubated with 40 μM of various fluorogenic peptide substrates in 100 μL assay buffer (20 mM Tris-HCl ,pH 7.5), in the presence of Celastrol or Oridonin at different concentrations or in the solvent DMSO for 2 hours at 37°C, followed by measurement of inhibition of each proteasomal activity[1]. |
| Cell Assay | Prostate cancer cells (5,000-8,000) are plated in each well of a 96-well plate and then treated with either DMSO, Tripterin (Celastrol), or Oridonin at different concentrations for 12 to 16 hours, followed by an additional 2-hour incubation with Z-Gly-Gly-Leu-AMC (at 40 μM). After that, the proteasome activity is measured using the whole plate[1]. |
| Animal Admin | Mice[1] Male nude immunodeficient mice NCRNU-M, aged 5 weeks, are used. On day 0, human prostate cancer PC-3 or C4-2B cells (5-10×106) suspended in 0.1 mL of serum-free RPMI 1640 are inoculated s.c. in the right flank of each mouse (four mice per group). For the first experiment using PC-3 cells, on day 14 after inoculation, the animals started daily i.p. injection with either 50 to 100 μL of a vehicle [10% DMSO, 70% Cremophor/ethanol (3:1), and 20% PBS], and 1.0 or 3.0 mg/kg of Tripterin (Celastrol) . Tumor sizes are measured daily using calipers and their volumes are calculated using a standard formula: width2×length/2. Body weight is measured weekly. To study whether the proteasome is inhibited in an early phase of the experiment, after 3 days of treatment, one control and one 3.0 mg/kg Tripterin (Celastrol) -treated mouse is sacrificed. The rest are sacrificed after 16 days of treatment when control tumors reach 1,400 mm3. For the second PC-3 tumor experiment, 12 days after inoculation, mice are randomly divided into three groups and treated with either control, Tripterin (Celastrol) , or Oridonin at 1.5 mg/kg daily for the duration of the study (31 days). In another experiment, to study the effects of Tripterin (Celastrol) on AR expression, nude mice bearing C4-2B tumors receive daily i.p. injection of the vehicle or 3.0 mg/kg Tripterin (Celastrol) . Rats[2] Male Sprague-Dawley (SD) rats (n=90, 6 weeks old), weighing 161±9 g, are randomly divided into the control (NC) and the high energy diet (HED) groups. In the control group, the animals receive a standard chow diet, while the rats in the HED group are fed with an additional high energy emulsion. After 8 weeks on their respective diets, Streptozotocin (STZ; 45 mg/kg) dissolved in 0.1 mol/l citrate buffer (pH 4.5) is injected into the caudal vein of the rats in the HED group to establish a model of T2DM, while the rats in the control group are injected with sodium citrate buffer. The rats with blood glucose levels ≥16.7 mM at 7 days after the STZ injection are selected as the model of diabetes. On average, 80% of the rats injected with STZ met these criteria. At 1 week following the injection of STZ, the rats with successfully-induced diabetes are randomly divided into the diabetes model (DM) group, the Tripterin (Celastrol) low-dose group (1 mg/kg/day), the Tripterin (Celastrol) middle-dose group (3 mg/kg/day) and the Tripterin (Celastrol) high-dose group (6 mg/kg/day) (n=15 rats per group). The rats in the treatment groups are administered Tripterin (Celastrol) by gavage, whereas the rats in the NC and DM groups are administered an equal amount of distilled water (2 mL). Following 8 weeks of the respective treatments, rats are anesthetized with an intraperitoneal injection of sodium pentobarbital (30 mg/kg body weight) and tissue samples are collected for analysis. The paravertebral muscle is excised from the rat bodies, and is cut perpendicularly along the longitudinal axis and fixed in phosphate-buffered 20% formaldehyde. Histological paraffin-embedded sections (5 µm) are then prepared for H&E staining. The sections of paravertebral muscle are snap-frozen in liquid nitrogen and stored at −80°C until further analysis. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 645.7±55.0 °C at 760 mmHg |
| Melting Point | 185-200ºC |
| Molecular Formula | C29H38O4 |
| Molecular Weight | 450.610 |
| Flash Point | 358.3±28.0 °C |
| Exact Mass | 450.277008 |
| PSA | 74.60000 |
| LogP | 7.08 |
| Vapour Pressure | 0.0±4.4 mmHg at 25°C |
| Index of Refraction | 1.602 |
| Storage condition | Store at -20°C |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 2942000000 |
|---|
|
Biological activity and safety of Tripterygium extract prepared by sodium carbonate extraction.
Molecules 17(9) , 11113-23, (2012) The commercial preparation named “Tripterygium glycosides” prepared by column chromatography has been used for the treatment of inflammatory and autoimmune diseases with significant efficacy but concu... |
|
|
Inhibitory mechanisms of celastrol on human liver cytochrome P450 1A2, 2C19, 2D6, 2E1 and 3A4.
Xenobiotica 45 , 571-7, (2015) 1. The present study was conducted to examine the possibility of herb-drug interaction by celastrol, which is a main compound isolated from Tripterygium wilfordii Hook F. using human liver microsomes ... |
|
|
[Studies on sustained release solid dispersion of tripterine carried by HPMC-stearic acid].
Zhongguo Zhong Yao Za Zhi 37(20) , 3052-5, (2012) To prepare the sustained release solid dispersion of tripterine, using HPMC-stearic acid with the intention of improving drug dissolution and controlling drug releases moderate, so that the drug perfo... |
| Celasterol |
| (2R,4aS,6aS,12bR,14aS,14bR)-10-Hydroxy-2,4a,6a,9,12b,14a-hexamethyl-11-oxo-1,2,3,4,4a,5,6,6a,11,12b,13,14,14a,14b-tetradecahydro-2-picenecarboxylic acid |
| Celastrol |
| 2-Picenecarboxylic acid, 1,2,3,4,4a,5,6,6a,11,12b,13,14,14a,14b-tetradecahydro-10-hydroxy-2,4a,6a,9,12b,14a-hexamethyl-11-oxo-, (2R,4aS,6aS,12bR,14aS,14bR)- |
| Tripterine |
| (2R,4aS,6aS,12bR,14aS,14bR)-10-Hydroxy-2,4a,6a,9,12b,14a-hexamethyl-11-oxo-1,2,3,4,4a,5,6,6a,11,12b,13,14,14a,14b-tetradecahydropicene-2-carboxylic acid |
| MFCD03424073 |
| TRIPTERIN |
 CAS#:1258-84-0
CAS#:1258-84-0
