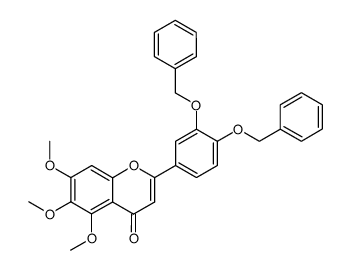
Cirsiliol
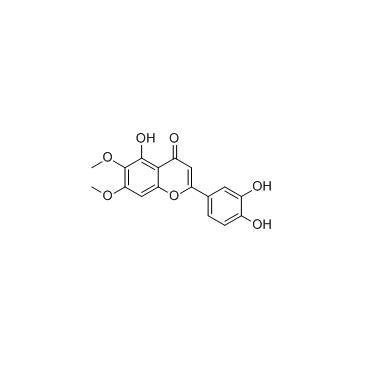
Cirsiliol structure
|
Common Name | Cirsiliol | ||
|---|---|---|---|---|
| CAS Number | 34334-69-5 | Molecular Weight | 330.289 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 616.1±55.0 °C at 760 mmHg | |
| Molecular Formula | C17H14O7 | Melting Point | 280-281.5℃ (methanol ) | |
| MSDS | Chinese USA | Flash Point | 230.8±25.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of CirsiliolCirsiliol is a potent and selective 5-lipoxygenase inhibitor and a competitive low affinity benzodiazepine receptor ligand. |
| Name | cirsiliol |
|---|---|
| Synonym | More Synonyms |
| Description | Cirsiliol is a potent and selective 5-lipoxygenase inhibitor and a competitive low affinity benzodiazepine receptor ligand. |
|---|---|
| Related Catalog | |
| Target |
Target: 5-lipoxygenase[1] |
| In Vitro | In concentrations from 0.01 to 300 μM, cirsiliol causes concentration-dependent relaxation of rat isolated ileum. Cirsiliol may inhibit Ca2+ influx but stimulates Ca2+ release from intracellular stores[1]. Treatment with rhamnetin or cirsiliol reduces the proliferation of NSCLC cells through the suppression of radiation-induced Notch-1 expression[2]. |
| In Vivo | In xenograft mouse model, tumor volume is significantly reduced by combinational treatment with irradiation and rhamnetin or cirsiliol compared with irradiation alone[2]. |
| Cell Assay | NSCLC, NCI-H1299, NCI-H460, WI-26 VA4 and MRC-5 cell lines are exposed to a single dose of γ-rays. Cells are then treated with rhamnetin and cirsiliol (5, 10, 15, 20, 25 μM) dissolved in DMSO for 4 h[2]. |
| Animal Admin | Mice[1] BALB/c athymic nude mice are injected with 2×106 NCI-H1299 cells. When the tumor has acquired a minimal volume of 200 mm3, DMSO or Cirsiliol (200 μg/kg body weight) is administered intraperitoneally every day for 25 days. The animals are also irradiated with 10 Gy once a week for 3 weeks. On day 25, the tumors are excised and subjected to further analyses[1]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 616.1±55.0 °C at 760 mmHg |
| Melting Point | 280-281.5℃ (methanol ) |
| Molecular Formula | C17H14O7 |
| Molecular Weight | 330.289 |
| Flash Point | 230.8±25.0 °C |
| Exact Mass | 330.073944 |
| PSA | 109.36000 |
| LogP | 2.27 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.671 |
| Storage condition | 2-8℃ |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
[Chemical constituents of Eupatorium lindleyanum].
Zhongguo Zhong Yao Za Zhi 37(7) , 937-40, (2012) To study chemical constituents of Eupatorium lindleyanum. Ethyl acetate extractive fractions were separated with silica gel and Sephadex LH-20 by column chromatography, and their structures were ident... |
|
|
[Determination of flavonoids in buds of Herba Artemisiae Scopariae by HPLC].
Zhongguo Zhong Yao Za Zhi 30(8) , 591-4, (2005) To develop a quantitative method for the determination of four flavonoids in buds of Herba Artemisiae Scopariae.The sample was extracted by ultrasonic with ethyl acetate for 30 minutes and separated o... |
|
|
Potent and selective 5-lipoxygenase inhibitors: cirsiliol and AA-861.
Adv. Prostaglandin. Thromboxane. Leukot. Res. 15 , 217-9, (1985)
|
| 5,3',4'-Trihydroxy-6,7-dimethoxyflavone |
| 2-(3,4-dihydroxyphenyl)-5-hydroxy-6,7-dimethoxychromen-4-one |
| 6,7-dimethoxy-5,3',4'-trihydroxyflavone |
| 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-5-hydroxy-6,7-dimethoxy- |
| 3',4',5-Trihydoxy-6,7-dimethoxyflavone |
| 2-(3,4-Dihydroxyphenyl)-5-hydroxy-6,7-dimethoxy-4H-chromen-4-one |
| 6-Hydroxyluteolin-6,7-dimethyl ether |
| 3',4',5-Trihydroxy-6,7-dimethoxyflavone |
| Cirsiliol |