5-Bromoindanone

5-Bromoindanone structure
|
Common Name | 5-Bromoindanone | ||
|---|---|---|---|---|
| CAS Number | 34598-49-7 | Molecular Weight | 211.06 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 303.7±31.0 °C at 760 mmHg | |
| Molecular Formula | C9H7BrO | Melting Point | 126-129 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 120.2±12.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 5-Bromoindanone5-Bromo-2,3-dihydro-1H-inden-1-one is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 5-bromo-2,3-dihydroinden-1-one |
|---|---|
| Synonym | More Synonyms |
| Description | 5-Bromo-2,3-dihydro-1H-inden-1-one is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 303.7±31.0 °C at 760 mmHg |
| Melting Point | 126-129 °C(lit.) |
| Molecular Formula | C9H7BrO |
| Molecular Weight | 211.06 |
| Flash Point | 120.2±12.2 °C |
| Exact Mass | 209.968018 |
| PSA | 17.07000 |
| LogP | 2.86 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.623 |
| Storage condition | -20°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H312-H315-H319-H332-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S26-S36-S36/37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2914700090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914700090 |
|---|---|
| Summary | HS: 2914700090 halogenated, sulphonated, nitrated or nitrosated derivatives of ketones and quinones, whether or not with other oxygen function Tax rebate rate:9.0% Supervision conditions:none VAT:17.0% MFN tariff:5.5% General tariff:30.0% |
|
Discovery of a series of imidazo[4,5-b]pyridines with dual activity at angiotensin II type 1 receptor and peroxisome proliferator-activated receptor-γ.
J. Med. Chem. 54 , 4219-4233, (2011) Mining of an in-house collection of angiotensin II type 1 receptor antagonists to identify compounds with activity at the peroxisome proliferator-activated receptor-γ (PPARγ) revealed a new series of ... |
|
|
Novel imidazolyl and triazolyl substituted biphenyl compounds: synthesis and evaluation as nonsteroidal inhibitors of human 17alpha-hydroxylase-C17, 20-lyase (P450 17).
Bioorg. Med. Chem. 8(6) , 1245-52, (2000) The synthesis of a new series of P450 17 inhibitors is described. The imidazol-1-yl compounds 5 showed strong inhibition of P450 17 rat and especially human enzyme, the most active compounds being 5ax... |
|
|
The identification of potent and selective imidazole-based inhibitors of B-Raf kinase.
Bioorg. Med. Chem. Lett. 16(2) , 378-381, (2006) A novel triarylimidazole derivative, SB-590885 (33), bearing a 2,3-dihydro-1H-inden-1-one oxime substituent has been identified as a potent and extremely selective inhibitor of B-Raf kinase. |
| 5-Bromoindan-1-one |
| 5-Bromo-1-indanone |
| 1H-Inden-1-one, 5-bromo-2,3-dihydro- |
| 1-Indanone, 5-bromo- |
| 1H-Inden-1-one,5-bromo-2,3-dihydro |
| 1-Indanone,5-bromo |
| 5-bromoindane-1-one |
| 5-Bromindan-1-on |
| 5-bromo-2,3-dihydro-1H-indene-1-one |
| MFCD00082718 |
| 5-Brom-2,3-dihydro-1H-inden-1-on |
| 5-bromo-2,3-dihydro-1H-inden-1-one |
| 5-bromo-indanone |
| 5-Bromoindanone |
 CAS#:31736-73-9
CAS#:31736-73-9 CAS#:42287-90-1
CAS#:42287-90-1 CAS#:335159-82-5
CAS#:335159-82-5 CAS#:83-33-0
CAS#:83-33-0 CAS#:3132-99-8
CAS#:3132-99-8 CAS#:15852-73-0
CAS#:15852-73-0 CAS#:107558-73-6
CAS#:107558-73-6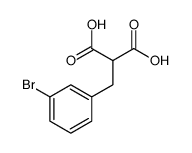 CAS#:125115-01-7
CAS#:125115-01-7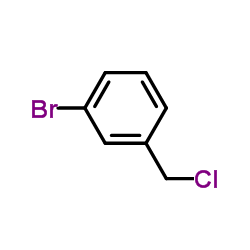 CAS#:932-77-4
CAS#:932-77-4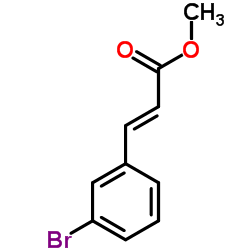 CAS#:3650-77-9
CAS#:3650-77-9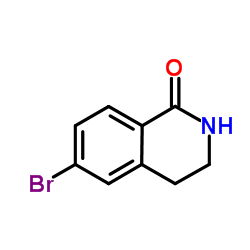 CAS#:147497-32-3
CAS#:147497-32-3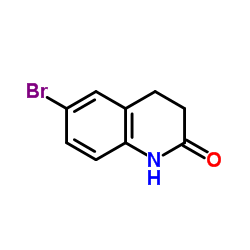 CAS#:3279-90-1
CAS#:3279-90-1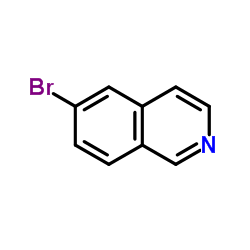 CAS#:34784-05-9
CAS#:34784-05-9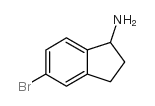 CAS#:185122-74-1
CAS#:185122-74-1 CAS#:33065-61-1
CAS#:33065-61-1 CAS#:75476-78-7
CAS#:75476-78-7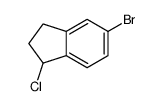 CAS#:158330-91-7
CAS#:158330-91-7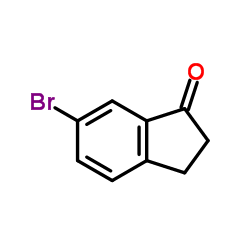 CAS#:14548-39-1
CAS#:14548-39-1 CAS#:338989-48-3
CAS#:338989-48-3 CAS#:34598-50-0
CAS#:34598-50-0
