Metiamide
Modify Date: 2024-01-11 15:10:02
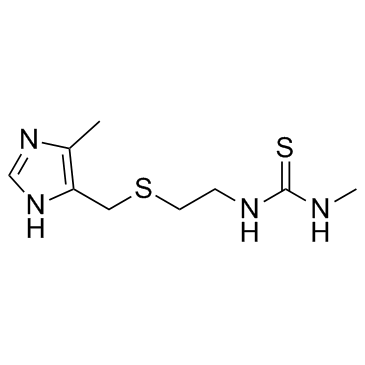
Metiamide structure
|
Common Name | Metiamide | ||
|---|---|---|---|---|
| CAS Number | 34839-70-8 | Molecular Weight | 244.380 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 463.0±55.0 °C at 760 mmHg | |
| Molecular Formula | C9H16N4S2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 233.8±31.5 °C | |
Use of MetiamideMetiamide is a histamine H2-receptor antagonist developed from another H2 antagonist, burimamide.IC50 Value: 0.92 uM (Ki with glycolaldehyde as the varied substrate for E3)Target: H2 receptorMetiamide is an intermediate compound in the development of the successful anti-ulcer drug cimetidine. in vitro: Metiamide is a competitive with aldehyde substrates and noncompetitive with the Human E3 Aldehyde Dehydrogenase coenzyme, binding to both the free E3 isozyme and the enzyme·coenzyme binary complex withK i values of 0.92 μM glycolaldehyde as the varied substrate[1]. Data was got as percentage change in GTPase activity induced by metiamide compared with the GTPase activity stimulated by HA (100 μM)[2]. in vivo: Metiamide is a histamine H2-receptor antagonist. It reduces basal and nocturnal gastric acid secretion and a reduction in gastric volume, acidity, and amount of gastric acid released in response to stimuli including food, caffeine, insulin, betazole, or pentagastrin. Metiamide inhibits many of the isoenzymes of the hepatic CYP450 enzyme system. Other actions of Metiamide include an increase in gastric bacterial flora such as nitrate-reducing organisms. |
| Name | 1-methyl-3-[2-[(5-methyl-1H-imidazol-4-yl)methylsulfanyl]ethyl]thiourea |
|---|---|
| Synonym | More Synonyms |
| Description | Metiamide is a histamine H2-receptor antagonist developed from another H2 antagonist, burimamide.IC50 Value: 0.92 uM (Ki with glycolaldehyde as the varied substrate for E3)Target: H2 receptorMetiamide is an intermediate compound in the development of the successful anti-ulcer drug cimetidine. in vitro: Metiamide is a competitive with aldehyde substrates and noncompetitive with the Human E3 Aldehyde Dehydrogenase coenzyme, binding to both the free E3 isozyme and the enzyme·coenzyme binary complex withK i values of 0.92 μM glycolaldehyde as the varied substrate[1]. Data was got as percentage change in GTPase activity induced by metiamide compared with the GTPase activity stimulated by HA (100 μM)[2]. in vivo: Metiamide is a histamine H2-receptor antagonist. It reduces basal and nocturnal gastric acid secretion and a reduction in gastric volume, acidity, and amount of gastric acid released in response to stimuli including food, caffeine, insulin, betazole, or pentagastrin. Metiamide inhibits many of the isoenzymes of the hepatic CYP450 enzyme system. Other actions of Metiamide include an increase in gastric bacterial flora such as nitrate-reducing organisms. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 463.0±55.0 °C at 760 mmHg |
| Molecular Formula | C9H16N4S2 |
| Molecular Weight | 244.380 |
| Flash Point | 233.8±31.5 °C |
| Exact Mass | 244.081635 |
| PSA | 110.13000 |
| LogP | 0.65 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.627 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| HS Code | 2933290090 |
|---|
|
~% 
Metiamide CAS#:34839-70-8 |
| Literature: US3954982 A1, ; US 3954982 A |
|
~% 
Metiamide CAS#:34839-70-8 |
| Literature: US4000302 A1, ; US 4000302 A |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| metiamide |
| Thiourea, N-methyl-N'-[2-[[(5-methyl-1H-imidazol-4-yl)methyl]thio]ethyl]- |
| Metiamidum |
| Metiamida |
| SK&Methiamide |
| Thiourea, N-methyl-N'-(2-(((5-methyl-1H-imidazol-4-yl)methyl)thio)ethyl)- |
| 1-Methyl-3-(2-{[(4-methyl-1H-imidazol-5-yl)methyl]sulfanyl}ethyl)thiourea |
![2-[[(5-methyl-1H-imidazol-4-yl)methyl]thio]ethylamine dihydrochloride structure](https://image.chemsrc.com/caspic/467/38603-72-4.png)
![4-[[(2-aminoethyl)thio]methyl]-5-methylimidazole structure](https://image.chemsrc.com/caspic/300/38585-67-0.png)