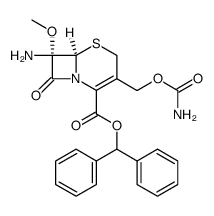
Cefoxitin
Cefoxitin structure
|
Common Name | Cefoxitin | ||
|---|---|---|---|---|
| CAS Number | 35607-66-0 | Molecular Weight | 427.452 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 843.4±65.0 °C at 760 mmHg | |
| Molecular Formula | C16H17N3O7S2 | Melting Point | 149 - 150ºC | |
| MSDS | N/A | Flash Point | 463.9±34.3 °C | |
Use of CefoxitinCefoxitin is a broad-spectrum, second-generation cephalosporin with antibacterial activity. Cefoxitin is effective against a wide variety of infections caused by gram-positive or gram-negative aerobes as well as by anaerobic bacteria[1][2]. |
| Name | cefoxitin |
|---|---|
| Synonym | More Synonyms |
| Description | Cefoxitin is a broad-spectrum, second-generation cephalosporin with antibacterial activity. Cefoxitin is effective against a wide variety of infections caused by gram-positive or gram-negative aerobes as well as by anaerobic bacteria[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Cefoxitin has good activity against gram-positive bacteria. The MICs for several gram-positive pathogens are in the range of 1-6 μg/mL[1]. Cefoxitin has shown high efficacy killing B. burgdorferi at concentration of 1.25 µM/mL[3]. |
| In Vivo | Cefoxitin (20 mg/kg; i.p.; daily; for 5 days) effectively kills B. burgdorferi in vivo C3H/HeN mouse model[3]. Animal Model: Four-week-old female C3H/HeN mice[3] Dosage: 20 mg/kg Administration: Intraperitoneal injection, daily, for five consecutive days Result: Had shown high efficacy killing B. burgdorferi in vivo. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 843.4±65.0 °C at 760 mmHg |
| Melting Point | 149 - 150ºC |
| Molecular Formula | C16H17N3O7S2 |
| Molecular Weight | 427.452 |
| Flash Point | 463.9±34.3 °C |
| Exact Mass | 427.050781 |
| PSA | 201.80000 |
| LogP | 0.63 |
| Vapour Pressure | 0.0±3.3 mmHg at 25°C |
| Index of Refraction | 1.693 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | UN 3077 |
|---|---|
| Packaging Group | III |
| HS Code | 3003201300 |
|
~56% 
Cefoxitin CAS#:35607-66-0 |
| Literature: Shiozaki, Masao; Ishida, Noboru; Iino, Kimio; Hiraoka, Tetsuo Tetrahedron, 1980 , vol. 36, p. 2735 - 2740 |
|
~% 
Cefoxitin CAS#:35607-66-0 |
| Literature: Tetrahedron, , vol. 36, p. 2735 - 2740 |
|
~% 
Cefoxitin CAS#:35607-66-0 |
| Literature: Tetrahedron, , vol. 36, p. 2735 - 2740 |
|
~% 
Cefoxitin CAS#:35607-66-0 |
| Literature: Tetrahedron, , vol. 36, p. 2735 - 2740 |
|
~% 
Cefoxitin CAS#:35607-66-0 |
| Literature: Tetrahedron, , vol. 36, p. 2735 - 2740 |
|
~% 
Cefoxitin CAS#:35607-66-0 |
| Literature: Tetrahedron, , vol. 36, p. 2735 - 2740 |
|
~% 
Cefoxitin CAS#:35607-66-0 |
| Literature: Tetrahedron, , vol. 36, p. 2735 - 2740 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
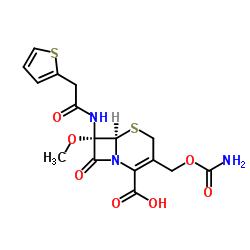
| 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[[(aminocarbonyl)oxy]methyl]-7-methoxy-8-oxo-7-[[2-(2-thienyl)acetyl]amino]-, (6R,7S)- |
| EINECS 252-641-2 |
| 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[[(aminocarbonyl)oxy]methyl]-7-methoxy-8-oxo-7-[(2-thienylacetyl)amino]-, (6R,7S)- |
| CEFOXITIN ACID |
| MFCD00072014 |
| Cefoxitin |
| Rephoxitin |
| (6R,7S)-3-[(Carbamoyloxy)methyl]-7-methoxy-8-oxo-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| CFX |
| (6R,7S)-3-{[(aminocarbonyl)oxy]methyl}-7-(methyloxy)-8-oxo-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| 3-Carbamoyloxymethyl-7a-methoxy-7-[2-(2-thienyl)acetamido]-3-cephem-4-carboxylic Acid |
| Mefoxin |
| cephoxitin |
| (6R,7S)-3-(Hydroxymethyl)-7-methoxy-8-oxo-7-(2-(2-thienyl)acetamido)-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid carbamate (ester) |
| (6R,7S)-3-[(carbamoyloxy)methyl]-7-methoxy-8-oxo-7-[(thiophen-2-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
![(6R)-3-carbamoyloxymethyl-7c-methoxy-8-oxo-7t-(2-thiophen-2-yl-acetylamino)-(6rH)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid benzhydryl ester structure](https://image.chemsrc.com/caspic/341/35607-68-2.png)
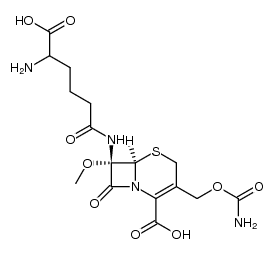


![2-(((6R,7S)-2-((benzhydryloxy)carbonyl)-3-((((carboxycarbonyl)carbamoyl)oxy)methyl)-7-methoxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-7-yl)amino)-2-oxoacetic acid structure](https://image.chemsrc.com/caspic/060/68318-53-6.png)