Benzbromarone
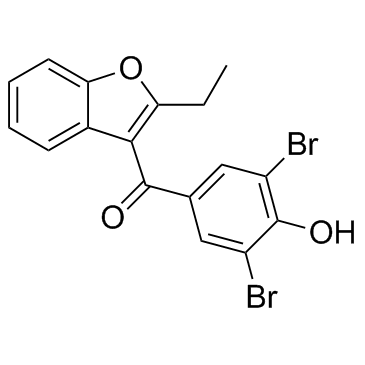
Benzbromarone structure
|
Common Name | Benzbromarone | ||
|---|---|---|---|---|
| CAS Number | 3562-84-3 | Molecular Weight | 424.083 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 514.1±50.0 °C at 760 mmHg | |
| Molecular Formula | C17H12Br2O3 | Melting Point | 161 - 163ºC | |
| MSDS | Chinese USA | Flash Point | 264.7±30.1 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of BenzbromaroneBenzbromarone is a highly effective and well tolerated non-competitive inhibitor of xanthine oxidase, used as an uricosuric agent, used in the treatment of gout. |
| Name | benzbromarone |
|---|---|
| Synonym | More Synonyms |
| Description | Benzbromarone is a highly effective and well tolerated non-competitive inhibitor of xanthine oxidase, used as an uricosuric agent, used in the treatment of gout. |
|---|---|
| Related Catalog |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 514.1±50.0 °C at 760 mmHg |
| Melting Point | 161 - 163ºC |
| Molecular Formula | C17H12Br2O3 |
| Molecular Weight | 424.083 |
| Flash Point | 264.7±30.1 °C |
| Exact Mass | 421.915314 |
| PSA | 50.44000 |
| LogP | 6.64 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.673 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | Missing Phrase - N15.00950417 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | UN 2811 |
| WGK Germany | 3 |
| RTECS | OB1804200 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2932190090 |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Interactions of urate transporter URAT1 in human kidney with uricosuric drugs.
Nephrology (Carlton.) 16(2) , 156-62, (2011) Hyperuricaemia is a significant factor in a variety of diseases, including gout and cardiovascular diseases. The kidney plays a dominant role in maintaining plasma urate levels through the excretion p... |
|
|
The uric acid transporter SLC2A9 is a direct target gene of the tumor suppressor p53 contributing to antioxidant defense.
Oncogene 34(14) , 1799-810, (2015) Only humans and higher primates have high uric acid blood levels. Although high uric acid causes gout, it has been linked with human longevity because of its hypothetical antioxidant function. Recent ... |
|
|
[Clinical strategies for prevention of drug-induced urinary calculi].
Clin. Calcium 21(10) , 1457-63, (2011) Drug-induced urinary calculi, although they account for only 1-2% of urinary calculi, deserve consideration because most of them are preventable. In the drug-containing calculi resulting from the crys... |
| Normurat |
| Methanone, (3,5-dibromo-4-hydroxyphenyl)(2-ethyl-3-benzofuranyl)- |
| (3,5-Dibromo-4-hydroxyphenyl)(2-ethyl-1-benzofuran-3-yl)methanone |
| EINECS 222-630-7 |
| 2-Ethyl-3-benzofuryl 3,5-Dibromo-4-hydroxyphenyl Ketone |
| (3,5-dibromo-4-hydroxyphenyl)(2-ethylbenzofuran-3-yl)methanone |
| 3-[3,5-DIBROMO-4-HYDROXYBENZOYL]-2-ETHYLBENZOFURAN |
| Minuric |
| Exurate |
| Hipurik |
| Benzbromarone |
| Desuric |
| Benzbromaron |
| Narcaricin |
| 3-(3,5-Dibromo-4-hydroxybenzoyl)-2-ethylbenzofuran |
| 2-Ethyl-3-benzofuranyl 4-Hydroxy-3,5-dibromophenyl Ketone |
| 3,5-Dibromo-4-hydroxyphenyl 2-Ethyl-3-benzofuranyl Ketone |
| (3,5-dibromo-4-hydroxyphenyl)-(2-ethyl-3-benzofuranyl) methanone |
| MFCD00078962 |
| 2-Ethyl-3-(3,5-dibromo-4-hydroxybenzoyl)benzofuran |
| (3,5-dibromo-4-hydroxyphenyl)-(2-ethyl-1-benzofuran-3-yl)methanone |
| Urinorm |
| Uricovac |
| (3,5-Dibromo-4-hydroxyphenyl)(2-ethyl-3-benzofuranyl)methanone |
| 2-Ethyl-3-(3,5-dibromo-4-hydroxybenzoyl)oxaindene |
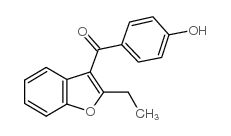 CAS#:1477-19-6
CAS#:1477-19-6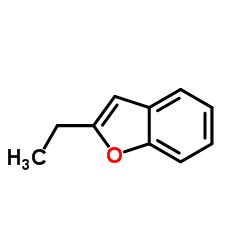 CAS#:3131-63-3
CAS#:3131-63-3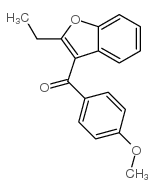 CAS#:3343-80-4
CAS#:3343-80-4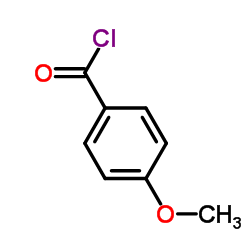 CAS#:100-07-2
CAS#:100-07-2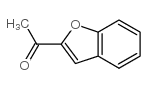 CAS#:1646-26-0
CAS#:1646-26-0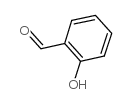 CAS#:90-02-8
CAS#:90-02-8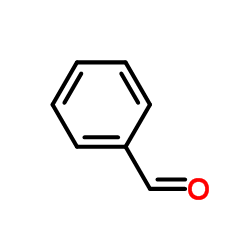 CAS#:100-52-7
CAS#:100-52-7 CAS#:51073-13-3
CAS#:51073-13-3
