Hexahydrocurcumin
Modify Date: 2024-01-02 23:12:34

Hexahydrocurcumin structure
|
Common Name | Hexahydrocurcumin | ||
|---|---|---|---|---|
| CAS Number | 36062-05-2 | Molecular Weight | 374.427 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 622.6±55.0 °C at 760 mmHg | |
| Molecular Formula | C21H26O6 | Melting Point | 80-82℃ | |
| MSDS | Chinese USA | Flash Point | 218.4±25.0 °C | |
Use of HexahydrocurcuminHexahydrocurcumin is a natural compound which posesses anticancer and anti-inflammatory activities; selective COX-2 inhibitor.IC50 value:Target: in vitro: The relative antioxidant potencies of ginger compounds decreased in similar order of 1-dehydro-[6]-gingerdione, hexahydrocurcumin>6-shogaol>6-dehydroshogaol in both 1,1-diphenyl-2-picyrlhydrazyl (DPPH) radical-scavenging and trolox equivalent antioxidant capacity (TEAC) assays [1]. HHC alone markedly decreased the viability of HT-29 human colon cancer cells compared to control. HHC significantly down-regulates COX-2 mRNA expression compared to the control (control: 100.05% ± 0.03% vs HHC: 61.01% ± 0.35%, P < 0.05) but does not alter COX-1 mRNA. In combined treatment, addition of HHC to a low dose of 5-FU exerts a synergistic effect against the growth of HT-29 cells by markedly reducing cell viability to a greater degree than monotherapy [2].in vivo: The combined effects of HHC with 5-FU exhibit a synergistic inhibition by decreasing ACF formation mediated by down-regulation of COX-2 expression in rats [3]. |
| Name | Hexahydrocurcumin |
|---|---|
| Synonym | More Synonyms |
| Description | Hexahydrocurcumin is a natural compound which posesses anticancer and anti-inflammatory activities; selective COX-2 inhibitor.IC50 value:Target: in vitro: The relative antioxidant potencies of ginger compounds decreased in similar order of 1-dehydro-[6]-gingerdione, hexahydrocurcumin>6-shogaol>6-dehydroshogaol in both 1,1-diphenyl-2-picyrlhydrazyl (DPPH) radical-scavenging and trolox equivalent antioxidant capacity (TEAC) assays [1]. HHC alone markedly decreased the viability of HT-29 human colon cancer cells compared to control. HHC significantly down-regulates COX-2 mRNA expression compared to the control (control: 100.05% ± 0.03% vs HHC: 61.01% ± 0.35%, P < 0.05) but does not alter COX-1 mRNA. In combined treatment, addition of HHC to a low dose of 5-FU exerts a synergistic effect against the growth of HT-29 cells by markedly reducing cell viability to a greater degree than monotherapy [2].in vivo: The combined effects of HHC with 5-FU exhibit a synergistic inhibition by decreasing ACF formation mediated by down-regulation of COX-2 expression in rats [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 622.6±55.0 °C at 760 mmHg |
| Melting Point | 80-82℃ |
| Molecular Formula | C21H26O6 |
| Molecular Weight | 374.427 |
| Flash Point | 218.4±25.0 °C |
| Exact Mass | 374.172943 |
| PSA | 96.22000 |
| LogP | 1.49 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.583 |
| Storage condition | -20°C |
| RIDADR | NONH for all modes of transport |
|---|---|
| HS Code | 2914509090 |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
| Hexahydrocurcumin |
| 5-Hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)heptan-3-one |
| 5-Hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)-3-heptanone |
| (RS)-5-Hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)-3-heptanone |
| 3-Heptanone, 5-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)- |
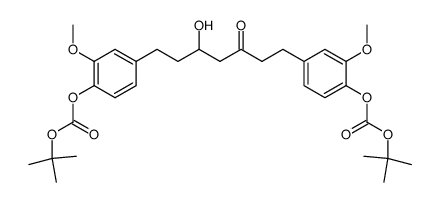 CAS#:869101-60-0
CAS#:869101-60-0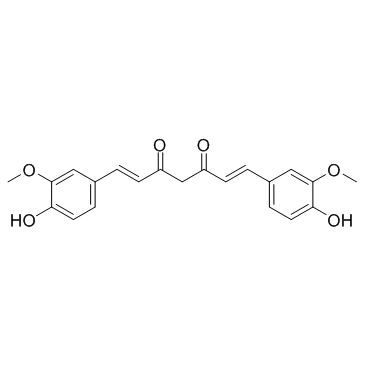 CAS#:458-37-7
CAS#:458-37-7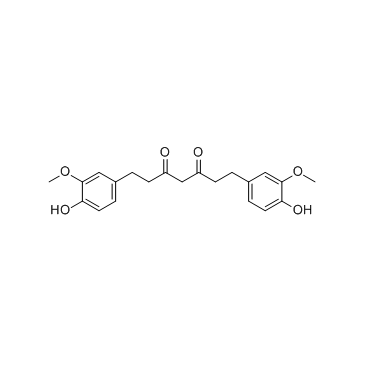 CAS#:36062-04-1
CAS#:36062-04-1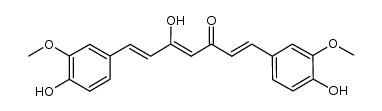 CAS#:147556-16-9
CAS#:147556-16-9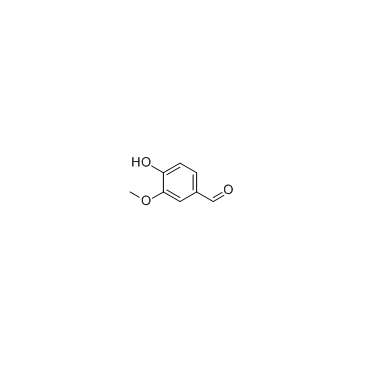 CAS#:121-33-5
CAS#:121-33-5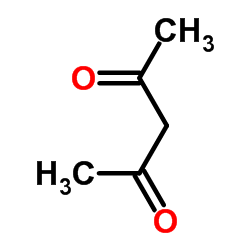 CAS#:123-54-6
CAS#:123-54-6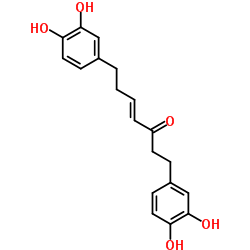 CAS#:41137-87-5
CAS#:41137-87-5
