CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
DV1761000
-
CHEMICAL NAME :
-
(1,1'-Biphenyl)-4-butanoic acid, gamma-oxo-
-
CAS REGISTRY NUMBER :
-
36330-85-5
-
BEILSTEIN REFERENCE NO. :
-
2378560
-
LAST UPDATED :
-
199703
-
DATA ITEMS CITED :
-
17
-
MOLECULAR FORMULA :
-
C16-H14-O3
-
MOLECULAR WEIGHT :
-
254.30
-
WISWESSER LINE NOTATION :
-
QV2VR DR
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
2314 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Blood - aplastic anemia
-
REFERENCE :
-
BJRHDF British Journal of Rheumatology. (Bailliere Tindall, 33 The Avenue, Eastborn BN21 3UN, UK) Volume(issue)/page/year: 33,389,1994
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
12 mg/kg/1D-I
-
TOXIC EFFECTS :
-
Behavioral - hallucinations, distorted perceptions
-
REFERENCE :
-
BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 290,822,1985
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
90 mg/kg/1W-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - cough Skin and Appendages - sweating Nutritional and Gross Metabolic - body temperature increase
-
REFERENCE :
-
HUTODJ Human Toxicology. (Macmillan Press Ltd., Houndmills, Basingstoke, Hants., RG 21 2XS, UK) V.1- 1981- Volume(issue)/page/year: 7,35,1988
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
180 mg/kg/10D-I
-
TOXIC EFFECTS :
-
Brain and Coverings - encephalitis Behavioral - convulsions or effect on seizure threshold Behavioral - coma
-
REFERENCE :
-
BJCPAT British Journal of Clinical Practice. (Medical News Group, 1 Bedford St., London WC2E 9HD, UK) V.10(10)- 1956- Volume(issue)/page/year: 48,277,1994
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
200 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from stomach Gastrointestinal - ulceration or bleeding from small intestine Blood - hemorrhage
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 30,721,1980
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
265 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from stomach Gastrointestinal - ulceration or bleeding from small intestine Blood - hemorrhage
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 30,721,1980
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
247 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from stomach Gastrointestinal - ulceration or bleeding from small intestine Blood - hemorrhage
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 30,721,1980
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
795 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from stomach Gastrointestinal - ulceration or bleeding from small intestine Blood - hemorrhage
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 30,721,1980
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
482 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
PCIPDV Beijing Yixueyuan Xuebao. Journal of Peking Medical College. (Beijing Yixueyuan, Beijiaohaidianqu, Beijing, Peop. Rep. China) 19??-V.17(3), 1985. Volume(issue)/page/year: 15,132,1983
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1189 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 10,884,1979 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2700 mg/kg/30D-I
-
TOXIC EFFECTS :
-
Blood - normocytic anemia Nutritional and Gross Metabolic - weight loss or decreased weight gain Related to Chronic Data - death
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 5,3511,1977
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
5776 mg/kg/91D-C
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from stomach Related to Chronic Data - death
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 30,721,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
16644 mg/kg/1Y-C
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from stomach Related to Chronic Data - death
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 30,721,1980 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
280 mg/kg
-
SEX/DURATION :
-
female 16-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Effects on Newborn - stillbirth Reproductive - Effects on Newborn - live birth index (measured after birth)
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 30,725,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1120 mg/kg
-
SEX/DURATION :
-
female 16-22 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 30,725,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
140 mg/kg
-
SEX/DURATION :
-
female 16-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 30,725,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
3780 mg/kg
-
SEX/DURATION :
-
male 63 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - male fertility index (e.g. # males impregnating females per # males exposed to fertile nonpregnant females)
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 30,725,1980
|
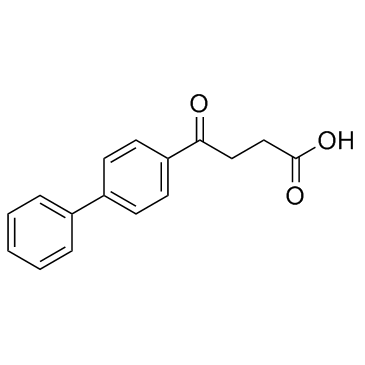


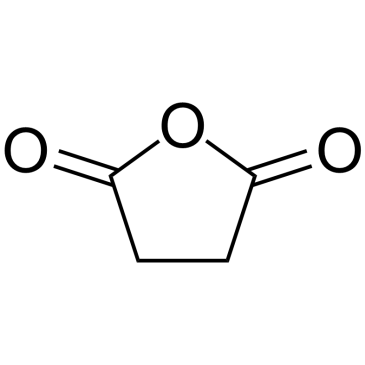 CAS#:108-30-5
CAS#:108-30-5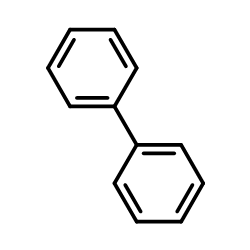 CAS#:92-52-4
CAS#:92-52-4 CAS#:1230-54-2
CAS#:1230-54-2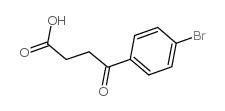 CAS#:6340-79-0
CAS#:6340-79-0 CAS#:143-66-8
CAS#:143-66-8 CAS#:35288-13-2
CAS#:35288-13-2 CAS#:92-66-0
CAS#:92-66-0 CAS#:14002-51-8
CAS#:14002-51-8 CAS#:7446-70-0
CAS#:7446-70-0 CAS#:98-95-3
CAS#:98-95-3 CAS#:6057-60-9
CAS#:6057-60-9 CAS#:141-82-2
CAS#:141-82-2 CAS#:92-92-2
CAS#:92-92-2 CAS#:124-38-9
CAS#:124-38-9 CAS#:3218-36-8
CAS#:3218-36-8 CAS#:63472-13-9
CAS#:63472-13-9 CAS#:63472-14-0
CAS#:63472-14-0 CAS#:40885-19-6
CAS#:40885-19-6
