CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
CV5375500
-
CHEMICAL NAME :
-
Benzamide, 2-hydroxy-5-(1-hydroxy-2-((1-methyl-3-phenylpropyl)am ino)ethyl)-
-
CAS REGISTRY NUMBER :
-
36894-69-6
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
7
-
MOLECULAR FORMULA :
-
C19-H24-N2-O3
-
MOLECULAR WEIGHT :
-
328.45
-
WISWESSER LINE NOTATION :
-
ZVR BQ EYQ1MY1&2R
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
8571 mg/kg/D
-
TOXIC EFFECTS :
-
Behavioral - changes in psychophysiological tests
-
REFERENCE :
-
BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 282,1824,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
240 mg/kg/14D
-
TOXIC EFFECTS :
-
Peripheral Nerve and Sensation - paresthesis
-
REFERENCE :
-
BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 1,580,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
2 mg/kg
-
TOXIC EFFECTS :
-
Skin and Appendages - dermatitis, allergic (after systemic exposure)
-
REFERENCE :
-
AIMEAS Annals of Internal Medicine. (American College of Physicians, 4200 Pine St., Philadelphia, PA 19104) V.1- 1927- Volume(issue)/page/year: 104,729,1986
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>2 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
PBPSDY Pharmacological and Biochemical Properties of Drug Substances. (American Pharmaceutical Assoc., 2215 Constitution Ave., NW, Washington, DC 20037) V.1- 1977- Volume(issue)/page/year: 2,229,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>50 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
PBPSDY Pharmacological and Biochemical Properties of Drug Substances. (American Pharmaceutical Assoc., 2215 Constitution Ave., NW, Washington, DC 20037) V.1- 1977- Volume(issue)/page/year: 2,229,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
660 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
USXXAM United States Patent Document. (U.S. Patent Office, Box 9, Washington, DC 20231) Volume(issue)/page/year: #5326774
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
97500 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
USXXAM United States Patent Document. (U.S. Patent Office, Box 9, Washington, DC 20231) Volume(issue)/page/year: #5326774
|

 CAS#:22374-89-6
CAS#:22374-89-6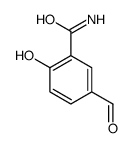 CAS#:76143-20-9
CAS#:76143-20-9
