Enmein
Modify Date: 2024-01-02 23:13:54
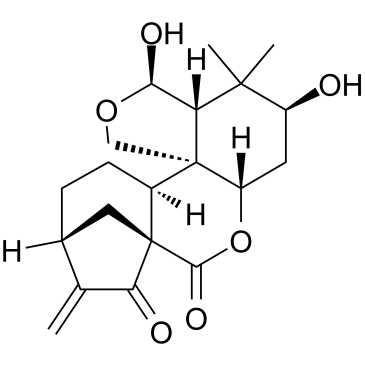
Enmein structure
|
Common Name | Enmein | ||
|---|---|---|---|---|
| CAS Number | 3776-39-4 | Molecular Weight | 362.417 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 610.3±55.0 °C at 760 mmHg | |
| Molecular Formula | C20H26O6 | Melting Point | 274-276℃ (Decomposition) (ethanol ) | |
| MSDS | N/A | Flash Point | 220.1±25.0 °C | |
Use of EnmeinEnmein is isolated from I. serra with immunosuppressive effect[1]. |
| Name | Enmein |
|---|---|
| Synonym | More Synonyms |
| Description | Enmein is isolated from I. serra with immunosuppressive effect[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Enmein shows significant inhibitory effect toward K562 with an IC50 value ranging from 3.2 μg/ml to 8.2 μg/ml[2]. Enmein (3.13-25 μg/ml; 48 hours) has inhibitory effect on lymphocytes with significant difference within concentration from 6.25 μg/ml to 25 μg/ml[1]. Enmein (5.5-22 μg/ml; 48 hours) causes the percentage of S stage cells decreased from 19% to 6%, causes the percentage of G2/M stage cells to decrease from 2% to 0.4%[1]. Cell Viability Assay[1] Cell Line: Splenic lymphocyte cells Concentration: 3.13, 6.25, 12.5, 25 μg/ml Incubation Time: 48 hours Result: Exhibited an IC50 value of 17 μg/ml in splenic lymphocytes. Cell Cycle Analysis[1] Cell Line: Splenic lymphocyte cells Concentration: 5.5 μg/ml, 11 μg/ml, 22 μg/ml Incubation Time: 48 hours Result: Caused S stage cells and G2/M stage cells decreased. |
| In Vivo | Enmein (inject to ear; 6.52-13.04 mg/kg; 5 days) inhibits the xylene-induced ear swelling and inhibits IL-2 expression[1]. Animal Model: Balb/c mice[1] Dosage: 6.52 mg/kg, 9.78 mg/kg or 13.04 mg/kg Administration: Injected to ear; 6.52 mg/kg, 9.78 mg/kg or 13.04 mg/kg; 5 days Result: Had inhibitory effect on IL-2 release in blood. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 610.3±55.0 °C at 760 mmHg |
| Melting Point | 274-276℃ (Decomposition) (ethanol ) |
| Molecular Formula | C20H26O6 |
| Molecular Weight | 362.417 |
| Flash Point | 220.1±25.0 °C |
| Exact Mass | 362.172943 |
| PSA | 93.06000 |
| LogP | -0.30 |
| Vapour Pressure | 0.0±4.0 mmHg at 25°C |
| Index of Refraction | 1.602 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| (1S,4S,6S,8R,9R,12S,13S,16R)-6,9-Dihydroxy-7,7-dimethyl-17-methylene-3,10-dioxapentacyclo[14.2.1.0.0.0]nonadecane-2,18-dione |
| 5H-5a,8-Methano-11H-cyclohepta[c]furo[3,4-e][1]benzopyran-5,6(7H)-dione, decahydro-2,13-dihydroxy-1,1-dimethyl-7-methylene-, (2S,3aS,5aS,8R,10aS,10bS,13R,13aR)- |
| 5H-5a,8-Methano-11H-cyclohepta(c)furo(3,4-e)(1)benzopyran-5,6(7H)-dione, decahydro-2,13-dihydroxy-1,1-dimethyl-7-methylene-, (2S,3as,5as,7R,8R,10aS,10bS,13R,13aR)- |
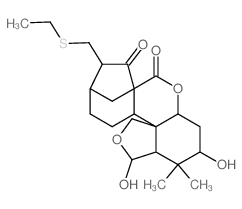 CAS#:81776-98-9
CAS#:81776-98-9
