Boc-Met-OSu
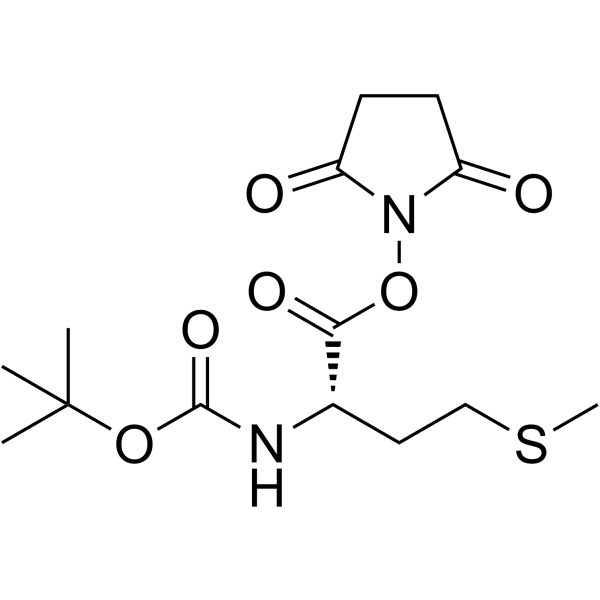
Boc-Met-OSu structure
|
Common Name | Boc-Met-OSu | ||
|---|---|---|---|---|
| CAS Number | 3845-64-5 | Molecular Weight | 346.399 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C14H22N2O6S | Melting Point | 120-126ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of Boc-Met-OSuBoc-Met-OSu is a Methionine (HY-13694) derivative[1]. |
| Name | boc-met-osu |
|---|---|
| Synonym | More Synonyms |
| Description | Boc-Met-OSu is a Methionine (HY-13694) derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Melting Point | 120-126ºC |
| Molecular Formula | C14H22N2O6S |
| Molecular Weight | 346.399 |
| Exact Mass | 346.119843 |
| PSA | 127.31000 |
| LogP | 0.63 |
| Appearance of Characters | Solid |
| Index of Refraction | 1.536 |
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 29309090 |
|
~67% 
Boc-Met-OSu CAS#:3845-64-5 |
| Literature: Appel, Rolf; Glaesel, Ursula Chemische Berichte, 1980 , vol. 113, # 11 p. 3511 - 3516 |
|
~91% 
Boc-Met-OSu CAS#:3845-64-5 |
| Literature: Jaoudai, Mahmoud; Martinez, Jean; Castro, Bertrand Journal of Organic Chemistry, 1987 , vol. 52, # 12 p. 2364 - 2367 |
|
~35% 
Boc-Met-OSu CAS#:3845-64-5 |
| Literature: Konnert, Laure; Lamaty, Frederic; Martinez, Jean; Colacino, Evelina Journal of Organic Chemistry, 2014 , vol. 79, # 9 p. 4008 - 4017 |
|
~% 
Boc-Met-OSu CAS#:3845-64-5 |
| Literature: Lyons, Anthony Q.; Pettit, Leslie D. Journal of the Chemical Society, Dalton Transactions: Inorganic Chemistry (1972-1999), 1984 , p. 2305 - 2308 |
| Precursor 5 | |
|---|---|
| DownStream 6 | |
|
High affinity binding of a glycopeptide elicitor to tomato cells and microsomal membranes and displacement by specific glycan suppressors.
J. Biol. Chem. 268(20) , 14724-31, (1993) We have previously isolated glycopeptides derived from yeast invertase that acted as highly potent elicitors in suspension-cultured tomato cells, inducing ethylene biosynthesis and phenylalanine ammon... |
|
|
Peracylation of nucleosides with methionine: foundation for a method to detect carcinogen adducts.
Chem. Res. Toxicol. 7(5) , 650-8, (1994) We report the chemical foundation for a new method to detect carcinogen-DNA adducts, which we have designated adduct detection by acylation with methionine (ADAM). The method is based on reaction of D... |
| BOC-L-METHIONINE N-HYDROXYSUCCINIMIDE ESTER |
| Boc-Met-OSu |
| N-(tert-Butoxycarbonyl)-L-Methionine N-SucciniMidyl Ester |
| N-Boc-L-methionine N-Succinimidyl Ester |
| BOC-Met-ONsu |
| BOC-METHIONINE-OSU |
| 2,5-Dioxo-1-pyrrolidinyl N-{[(2-methyl-2-propanyl)oxy]carbonyl}methioninate |
| n-hydroxysuccinimidylt-butoxycarbonylmethionine |
| BOC-L-METHIONINE HYDROXYSUCCINIMIDE ESTER |
| Methionine, N-[(1,1-dimethylethoxy)carbonyl]-, 2,5-dioxo-1-pyrrolidinyl ester |
| t-Boc-Met-OSu |
| N-tert-butyloxycarbonyl-L-methionine N-succinimidoyl ester |
| TBM-NHS |
| Boc-L-Met-OSu |
| Boc-L-methionine hydeoxysuccinimide ester |
| N-Boc-L-methionine N-Succinimidyl EsterBoc-Met-OSu |
| BOC-L-METHIONINE HYDROXYSUCCINIMIDESTER |
| tert-butyloxycarbonylmethionine N-hydroxysuccinimide ester |
| N-Boc-L-methionine N-hydroxysuccinimide ester |
 CAS#:23446-03-9
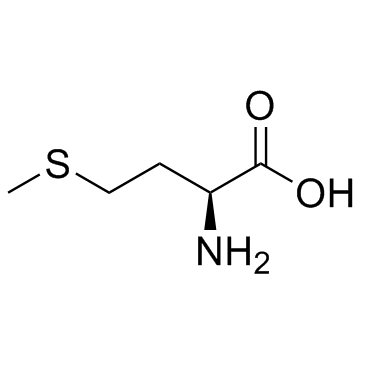
CAS#:23446-03-9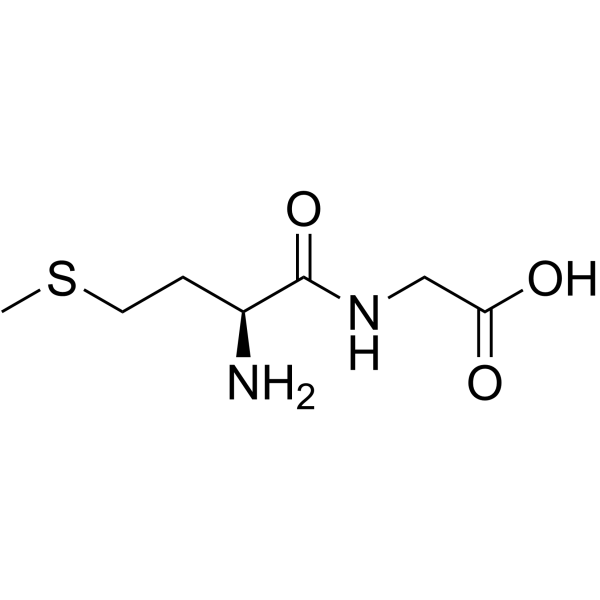 CAS#:14486-03-4
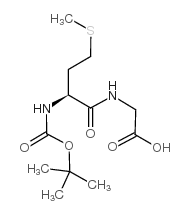
CAS#:14486-03-4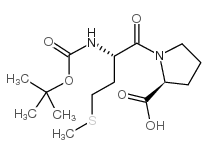 CAS#:116939-85-6
CAS#:116939-85-6![(2S)-2-[[(2S)-2-formamido-4-methylsulfanylbutanoyl]amino]-4-methylpentanoic acid structure](https://image.chemsrc.com/caspic/345/18321-99-8.png) CAS#:18321-99-8
CAS#:18321-99-8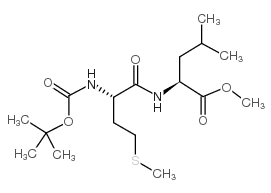 CAS#:66880-59-9
CAS#:66880-59-9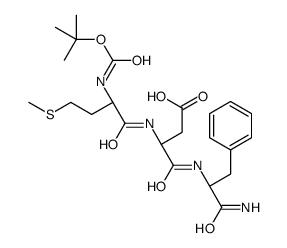 CAS#:5920-14-9
CAS#:5920-14-9
