Pasireotide L-aspartate salt
Modify Date: 2024-01-06 17:50:03
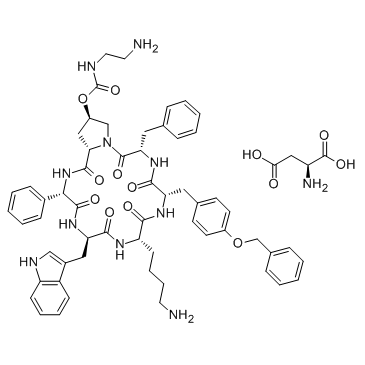
Pasireotide L-aspartate salt structure
|
Common Name | Pasireotide L-aspartate salt | ||
|---|---|---|---|---|
| CAS Number | 396091-77-3 | Molecular Weight | 1180.309 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C62H73N11O13 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Pasireotide L-aspartate saltPasireotide(SOM 230) is a stable cyclohexapeptide somatostatin mimic that exhibits unique high-affinity binding to human somatostatin receptors (subtypes sst1/2/3/4/5, pKi=8.2/9.0/9.1/<7.0/9.9 respectively).IC50 value: 8.2/9.0/9.1/<7.0/9.9(pKi, sst1/2/3/4/5) [1]in vitro: SOM230 showed a lower potency of GH release inhibition (IC(50), 0.5 nM), compared with OCT (IC(50), 0.02 nM) and SRIF-14 (IC(50), 0.02 nM). A positive correlation was found between sst(2) but not sst(5) mRNA levels in the adenoma cells and the inhibitory potency of OCT on GH release in vivo and in vitro, and the effects of SOM230 and SRIF-14 in vitro [2]. In cultures of human fetal pituitary cells, SOM230 inhibited GH secretion by 42 +/- 9% (P = 0.002) but had no effect on TSH release. SOM230 inhibited GH release from GH-secreting adenoma cultures by 34 +/- 8% (P = 0.002), PRL by 35 +/- 4% from PRL-secreting adenomas (P = 0.01), and alpha-subunit secretion from nonfunctioning pituitary adenomas by 46 +/- 18% (P = 0.34) [3].in vivo: On day 7, there was a decrease in serum insulin levels from 1.06 ± 0.28 μg/L to 0.37 ± 0.17 μg/L (P = .0128) and a significant increase in serum glucose from 4.2 ± 0.45 mmol/L to 7.12 ± 1.06 mmol/L (P = .0075) in the treatment group but no change in the control group [4]. In wild-type mice, both octreotide and pasireotide significantly attenuated knee joint swelling and histopathologic manifestations of arthritis to an extent comparable to that of dexamethasone. In SSTR2(-/-) mice, the antiinflammatory effects of both octreotide and pasireotide were completely abrogated [5]. |
| Name | Pasireotide L-aspartate salt |
|---|---|
| Synonym | More Synonyms |
| Description | Pasireotide(SOM 230) is a stable cyclohexapeptide somatostatin mimic that exhibits unique high-affinity binding to human somatostatin receptors (subtypes sst1/2/3/4/5, pKi=8.2/9.0/9.1/<7.0/9.9 respectively).IC50 value: 8.2/9.0/9.1/<7.0/9.9(pKi, sst1/2/3/4/5) [1]in vitro: SOM230 showed a lower potency of GH release inhibition (IC(50), 0.5 nM), compared with OCT (IC(50), 0.02 nM) and SRIF-14 (IC(50), 0.02 nM). A positive correlation was found between sst(2) but not sst(5) mRNA levels in the adenoma cells and the inhibitory potency of OCT on GH release in vivo and in vitro, and the effects of SOM230 and SRIF-14 in vitro [2]. In cultures of human fetal pituitary cells, SOM230 inhibited GH secretion by 42 +/- 9% (P = 0.002) but had no effect on TSH release. SOM230 inhibited GH release from GH-secreting adenoma cultures by 34 +/- 8% (P = 0.002), PRL by 35 +/- 4% from PRL-secreting adenomas (P = 0.01), and alpha-subunit secretion from nonfunctioning pituitary adenomas by 46 +/- 18% (P = 0.34) [3].in vivo: On day 7, there was a decrease in serum insulin levels from 1.06 ± 0.28 μg/L to 0.37 ± 0.17 μg/L (P = .0128) and a significant increase in serum glucose from 4.2 ± 0.45 mmol/L to 7.12 ± 1.06 mmol/L (P = .0075) in the treatment group but no change in the control group [4]. In wild-type mice, both octreotide and pasireotide significantly attenuated knee joint swelling and histopathologic manifestations of arthritis to an extent comparable to that of dexamethasone. In SSTR2(-/-) mice, the antiinflammatory effects of both octreotide and pasireotide were completely abrogated [5]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C62H73N11O13 |
|---|---|
| Molecular Weight | 1180.309 |
| Exact Mass | 1179.538940 |
| Storage condition | 2-8℃ |
| L-Aspartic acid, compd. with cyclo[(2S)-2-phenylglycyl-D-tryptophyl-L-lysyl-O-(phenylmethyl)-L-tyrosyl-L-phenylalanyl-(4R)-4-[[[(2-aminoethyl)amino]carbonyl]oxy]-L-prolyl] (1:1) |
| Pasireotide L-aspartate salt |
| L-Aspartic acid - cyclo[(2S)-2-phenylglycyl-D-tryptophyl-L-lysyl-O-benzyl-L-tyrosyl-L-phenylalanyl-(4R)-4-{[(2-aminoethyl)carbamoyl]oxy}-L-prolyl] (1:1) |
| Pasireotide (L-aspartate salt) |