Glycitein
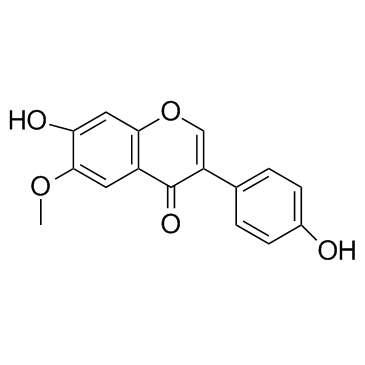
Glycitein structure
|
Common Name | Glycitein | ||
|---|---|---|---|---|
| CAS Number | 40957-83-3 | Molecular Weight | 284.263 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 547.4±50.0 °C at 760 mmHg | |
| Molecular Formula | C16H12O5 | Melting Point | >300ºC | |
| MSDS | Chinese USA | Flash Point | 210.1±23.6 °C | |
Use of GlyciteinGlycitein is a soybean (yellow cultivar) isoflavonoid; used in combination with other isoflavonoids such as genistein and daidzein to study apoptosis and anti-oxidation processes. |
| Name | glycitein |
|---|---|
| Synonym | More Synonyms |
| Description | Glycitein is a soybean (yellow cultivar) isoflavonoid; used in combination with other isoflavonoids such as genistein and daidzein to study apoptosis and anti-oxidation processes. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 547.4±50.0 °C at 760 mmHg |
| Melting Point | >300ºC |
| Molecular Formula | C16H12O5 |
| Molecular Weight | 284.263 |
| Flash Point | 210.1±23.6 °C |
| Exact Mass | 284.068481 |
| PSA | 79.90000 |
| LogP | 2.57 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.669 |
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2914509090 |
| Precursor 10 | |
|---|---|
| DownStream 3 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Isoflavone pharmacokinetics and metabolism after consumption of a standardized soy and soy-almond bread in men with asymptomatic prostate cancer.
Cancer Prev. Res. (Phila.) 8 , 1045-54, (2015) Epidemiologic associations suggest that populations consuming substantial amounts of dietary soy exhibit a lower risk of prostate cancer. A 20-week randomized, phase II, crossover trial was conducted ... |
|
|
Amino acid, mineral, and polyphenolic profiles of black vinegar, and its lipid lowering and antioxidant effects in vivo.
Food Chem. 168 , 63-9, (2014) Black vinegar (BV) contains abundant essential and hydrophobic amino acids, and polyphenolic contents, especially catechin and chlorogenic acid via chemical analyses. K and Mg are the major minerals i... |
|
|
Isoflavone extraction from okara using water as extractant.
Food Chem. 160 , 371-8, (2014) We here report on the use of water as a 'green' extraction solvent for the isolation of isoflavones from okara, a by-product of soymilk production. At a low liquid-to-solid ratio of 20 to 1 and 20 °C,... |
| 7-hydroxy-3-(4-hydroxyphenyl)-6-methoxychromen-4-one |
| 7-hydroxy-3-(4-hydroxyphenyl)-6-methoxy-4-chromenone |
| MFCD00016679 |
| Glycitein |
| Glycetein |
| 4',7-Dihydroxy-6-methoxyisoflavone |
| 7,4'-Dihydroxy-6-methoxyisoflavone |
| 4’,7-dihydroxy-6-methoxyisoflavone |
| 4,7-Dihydroxy-6-methoxyisoflavone |
| 7-Hydroxy-3-(4-hydroxyphenyl)-6-methoxy-4H-chromen-4-one |
| 4H-1-Benzopyran-4-one, 7-hydroxy-3-(4-hydroxyphenyl)-6-methoxy- |
| 7-Hydroxy-3-(4-hydroxyphenyl)-6-methoxy-4H-1-benzopyran-4-one |
| 4H-1-Benzopyran-4-one,7-hydroxy-3-(4-hydroxyphenyl)-6-methoxy |
 CAS#:124-63-0
CAS#:124-63-0 CAS#:79744-58-4
CAS#:79744-58-4 CAS#:58115-20-1
CAS#:58115-20-1 CAS#:68-12-2
CAS#:68-12-2 CAS#:67-56-1
CAS#:67-56-1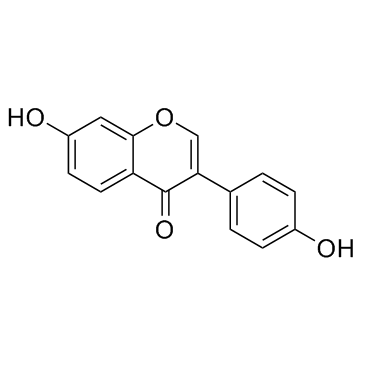 CAS#:486-66-8
CAS#:486-66-8![1-[5-Methoxy-2,4-bis(phenylmethoxy)phenyl]-ethanone Structure](https://image.chemsrc.com/caspic/390/7298-22-8.png) CAS#:7298-22-8
CAS#:7298-22-8![3,3-Dimethoxy-1-[5-Methoxy-1,4-bis(phenylmethoxy)phenyl]-2-[4-(phenylmethoxy)phenyl]-1-propanone Structure](https://image.chemsrc.com/caspic/342/58115-19-8.png) CAS#:58115-19-8
CAS#:58115-19-8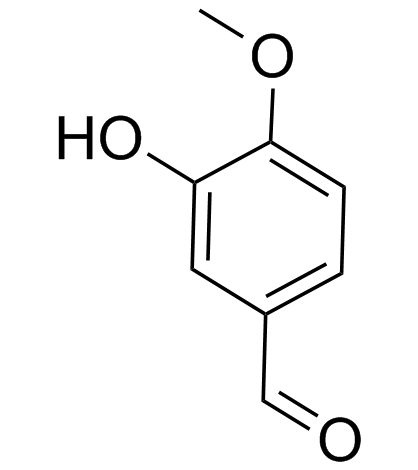 CAS#:621-59-0
CAS#:621-59-0 CAS#:19009-24-6
CAS#:19009-24-6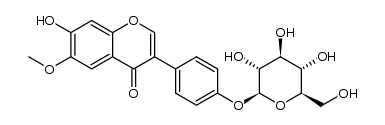 CAS#:1237605-01-4
CAS#:1237605-01-4 CAS#:40246-10-4
CAS#:40246-10-4 CAS#:17817-31-1
CAS#:17817-31-1
