TP2
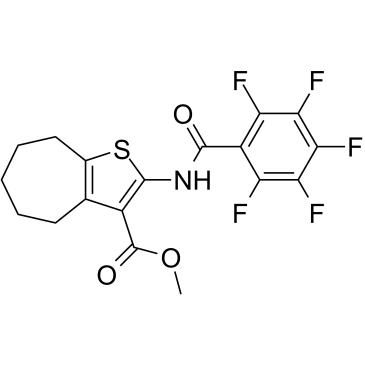
TP2 structure
|
Common Name | TP2 | ||
|---|---|---|---|---|
| CAS Number | 420089-51-6 | Molecular Weight | 419.37 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C18H14F5NO3S | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
Use of TP2Thiophene-2 (TP2) is a specific polyketide synthase 13 (Pks13) inhibitor. Thiophene-2 inhibits mycolic acid biosynthesis and rapidly leads to mycobacterial cell death. Thiophene-2 is active against Mycobacterium tuberculosis with a MIC value of 1 μM, and has potent anti-tuberculosis activity[1]. |
| Name | Thiophene-2 |
|---|---|
| Synonym | More Synonyms |
| Description | Thiophene-2 (TP2) is a specific polyketide synthase 13 (Pks13) inhibitor. Thiophene-2 inhibits mycolic acid biosynthesis and rapidly leads to mycobacterial cell death. Thiophene-2 is active against Mycobacterium tuberculosis with a MIC value of 1 μM, and has potent anti-tuberculosis activity[1]. |
|---|---|
| Related Catalog | |
| Target |
MIC: 1 μM (Mycobacterium tuberculosis) |
| In Vitro | In vitro, TP inhibits fatty acyl-AMP loading onto Pks13. Thiophene-2 (TP2; 0-125 μM) inhibits loading of wild-type Mycobacterium tuberculosis (Mtb) Pks13 (Pks13_WT) in a dose-dependent manner. Thiophene-2 also inhibits palmitic acid (FL C16) loading onto the TP-resistant F79S mutant protein[1]. Thiophene-2 has an IC50 versus monkey kidney Vero cells and human liver carcinoma HepG2 cells of 17.5 and 7.30 μM, respectively. Significant intracellular killing activity within BCG-infected J774A.1 macrophage cells is observed at Thiophene-2 concentrations of 12.8 μM[1]. |
| References |
| Molecular Formula | C18H14F5NO3S |
|---|---|
| Molecular Weight | 419.37 |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
|
Greater Sensitivity of Blood Pressure Than Renal Toxicity to Tyrosine Kinase Receptor Inhibition With Sunitinib.
Hypertension 66 , 543-9, (2015) Hypertension and renal injury are off-target effects of sunitinib, a tyrosine kinase receptor inhibitor used for the treatment of various tumor types. Importantly, these untoward effects are accompani... |
|
|
A method to quantitate the neutralizing capacity of anti-therapeutic protein antibodies in serum and their correlation to clinical impact.
J. Pharm. Biomed. Anal. 102 , 176-83, (2014) A robust, quantitative method for assessing the neutralizing capacity of anti-therapeutic protein antibodies was developed and tested using 4 analytical assay platforms typically used for detection of... |
|
|
Effect of a grain challenge on ruminal, urine, and fecal pH, apparent total-tract starch digestibility, and milk composition of Holstein and Jersey cows.
J. Dairy Sci. 99 , 2190-200, (2016) The effects of a grain challenge on ruminal, urine, and fecal pH, apparent total-tract starch digestibility, and milk composition were determined. Six Holstein cows, 6 rumen-cannulated Holstein cows, ... |
| MFCD01337175 |