Jervine

Jervine structure
|
Common Name | Jervine | ||
|---|---|---|---|---|
| CAS Number | 469-59-0 | Molecular Weight | 425.603 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 592.0±50.0 °C at 760 mmHg | |
| Molecular Formula | C27H39NO3 | Melting Point | 242- 244ºC | |
| MSDS | Chinese USA | Flash Point | 311.8±30.1 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of JervineJervine(11-Ketocyclopamine) is a naturally occuring steroidal alkaloid that causes cyclopia by blocking sonic hedgehog(Shh) signaling; Jervine is an inhibitor of Smo.IC50 value:Target: sonic hedgehog is derived from the Veratrum plant species. It is a close structural analog of cyclopamine which specifically inhibits the hedgehog (Hh) pathway by interaction with the hedgehog signaling protein Smo. Jervine can be used to induce abnormal morphogenesis in a number of experimental models. Jervine is an inhibitor of Smo. |
| Name | jervine |
|---|---|
| Synonym | More Synonyms |
| Description | Jervine(11-Ketocyclopamine) is a naturally occuring steroidal alkaloid that causes cyclopia by blocking sonic hedgehog(Shh) signaling; Jervine is an inhibitor of Smo.IC50 value:Target: sonic hedgehog is derived from the Veratrum plant species. It is a close structural analog of cyclopamine which specifically inhibits the hedgehog (Hh) pathway by interaction with the hedgehog signaling protein Smo. Jervine can be used to induce abnormal morphogenesis in a number of experimental models. Jervine is an inhibitor of Smo. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 592.0±50.0 °C at 760 mmHg |
| Melting Point | 242- 244ºC |
| Molecular Formula | C27H39NO3 |
| Molecular Weight | 425.603 |
| Flash Point | 311.8±30.1 °C |
| Exact Mass | 425.292999 |
| PSA | 58.56000 |
| LogP | 3.46 |
| Vapour Pressure | 0.0±3.8 mmHg at 25°C |
| Index of Refraction | 1.591 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | WG9700000 |
|
Development of an enzyme-linked immunosorbent assay for the veratrum plant teratogens: cyclopamine and jervine.
J. Agric. Food Chem. 51(3) , 582-6, (2003) Veratrum californicum was responsible for large losses of sheep grazing high mountain ranges in central Idaho in the 1950s. Veratrum induces various birth defects including the cyclopic-type craniofac... |
|
|
Cyclopamine and jervine induce COX-2 overexpression in human erythroleukemia cells but only cyclopamine has a pro-apoptotic effect.
Exp. Cell Res. 319(7) , 1043-53, (2013) Erythroleukemia is generally associated with a very poor response and survival to current available therapeutic agents. Cyclooxygenase-2 (COX-2) has been described to play a crucial role in the prolif... |
|
|
Effects of Veratrum nigrum alkaloids on central catecholaminergic neurons of renal hypertensive rats.
Acta Pharmacol. Sin. 21(1) , 23-8, (2000) To study the central hypotensive mechanism of Veratrum nigrum L var ussurience Nakai alkaloids (VnA) in renal hypertensive rats(RHR).The quantitative method of immunocytochemistry (ICC) was used to ob... |
| 17,23β-Epoxy-3β-hydroxyveratraman-11-one |
| (3β, 23β)-17,23-Epoxy-3-hydroxyveratraman-11-one |
| (3β,22S,23R)-3-Hydroxy-17,23-epoxyveratraman-11-one |
| Veratraman-11-one, 17,23-epoxy-3-hydroxy-, (3-β,23-β)- (9CI) |
| DL-ISOLEUCINE GRADE II |
| (3b,23b)-17,23-Epoxy-3-hydroxyveratraman-11-one |
| 4-27-00-03590 (Beilstein Handbook Reference) |
| (3β,23β)-17,23-Epoxy-3-hydroxyveratraman-11-one |
| Veratraman-11-one, 17,23-epoxy-3-hydroxy-, (3β,23β)- |
| 11-KETOCYCLOPAMINE |
| Iervin |
| Veratraman-11-one, 17,23-epoxy-3-hydroxy-, (3β,23β)- (9CI) |
| jerwiny |
| Jervin-11-one |
| Spiro[9H-benzo[a]fluorene-9,2'(3'H)-furo[3,2-b]pyridin]-11(1H)-one, 2,3,3'a,4,4',5',6,6',6a,6b,7,7',7'a,8,11a,11b-hexadecahydro-3-hydroxy-3',6',10,11b-tetramethyl-, (3S,3'R,3a'S,6'S,6aS,6bS,7a'R,9R,11aS,11bR)- |
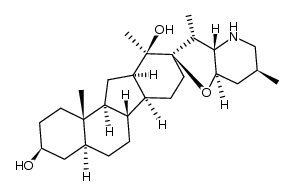 CAS#:17008-94-5
CAS#:17008-94-5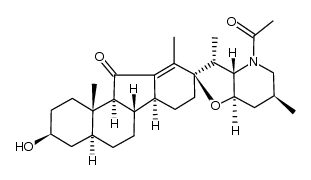 CAS#:17008-98-9
CAS#:17008-98-9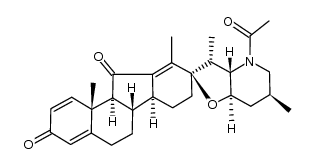 CAS#:17009-00-6
CAS#:17009-00-6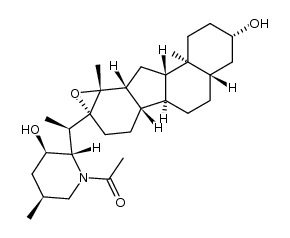 CAS#:17008-93-4
CAS#:17008-93-4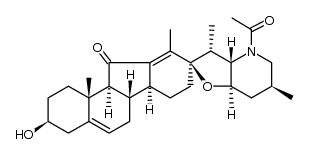 CAS#:17009-03-9
CAS#:17009-03-9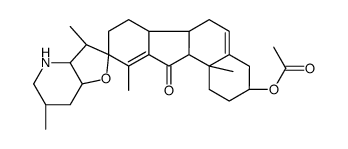 CAS#:14788-78-4
CAS#:14788-78-4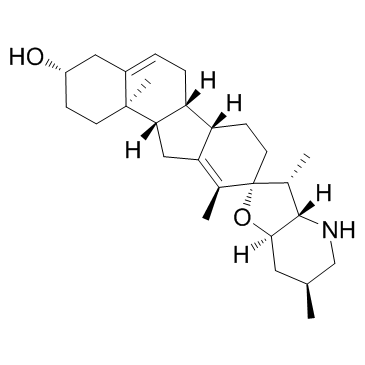 CAS#:4449-51-8
CAS#:4449-51-8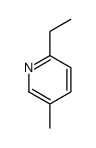 CAS#:18113-81-0
CAS#:18113-81-0
