5-Chloro-1-methyl-4-nitroimidazole
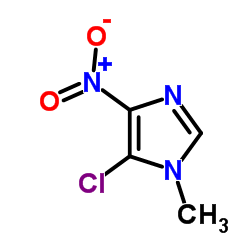
5-Chloro-1-methyl-4-nitroimidazole structure
|
Common Name | 5-Chloro-1-methyl-4-nitroimidazole | ||
|---|---|---|---|---|
| CAS Number | 4897-25-0 | Molecular Weight | 161.55 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 362.3±22.0 °C at 760 mmHg | |
| Molecular Formula | C4H4ClN3O2 | Melting Point | 148-150 °C(lit.) | |
| MSDS | USA | Flash Point | 172.9±22.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 5-Chloro-1-methyl-4-nitroimidazole5-Chloro-1-methyl-4-nitroimidazole is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 5-Chloro-1-methyl-4-nitroimidazole |
|---|---|
| Synonym | More Synonyms |
| Description | 5-Chloro-1-methyl-4-nitroimidazole is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 362.3±22.0 °C at 760 mmHg |
| Melting Point | 148-150 °C(lit.) |
| Molecular Formula | C4H4ClN3O2 |
| Molecular Weight | 161.55 |
| Flash Point | 172.9±22.3 °C |
| Exact Mass | 160.999207 |
| PSA | 63.64000 |
| LogP | 0.50 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.648 |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | NI4397100 |
| HS Code | 2933290090 |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
New synthesis and antiparasitic activity of model 5-aryl-1-methyl-4-nitroimidazoles.
Molecules 14(8) , 2758-67, (2009) A number of 5-aryl-1-methyl-4-nitroimidazoles 5a-f have been synthesized in good yields by the Suzuki coupling reaction between 5-chloro-1-methyl-4-nitroimidazole (3) and arylboronic acids 4a-f, aided... |
|
|
Rapid-mix studies on the anomalous radiosensitization of mammalian cells by 5-chloro-1-methyl-4-nitromidazole.
Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 38(6) , 673-5, (1980)
|
|
|
The kinetics of the reaction of 'anomalous' 4-nitroimidazole radiosensitizers with thiols.
Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 41(2) , 231-5, (1982)
|
| 1-methyl-5-chloro-4-nitroimidazole |
| PCMNI |
| 1-methyl-4-nitro-5-chloroimidazole |
| 5-chloro-1-methyl-4-nitro-imidazole |
| 5-Chloro-1-methyl-4-nitro-1H-imidazole |
| 5-Chloro-1-methyl-4-nitroimidazole |
| MFCD00233664 |
| 1H-Imidazole, 5-chloro-1-methyl-4-nitro- |
| 5-Chloro-1-methyl-4-nitroimidazole (AZATHIOPRINUM) |
| Imidazole,5-chloro-1-methyl-4-nitro |
| EINECS 225-521-2 |
| 1H-Imidazole,5-chloro-1-methyl-4-nitro |
| 1-METHYL-5-CHLORO-4-NITRO IMIDAZOLE |
 CAS#:872-49-1
CAS#:872-49-1 CAS#:19183-15-4
CAS#:19183-15-4 CAS#:186581-53-3
CAS#:186581-53-3 CAS#:57531-38-1
CAS#:57531-38-1 CAS#:77-78-1
CAS#:77-78-1 CAS#:7664-93-9
CAS#:7664-93-9 CAS#:4531-53-7
CAS#:4531-53-7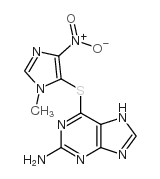 CAS#:5581-52-2
CAS#:5581-52-2 CAS#:35687-41-3
CAS#:35687-41-3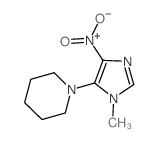 CAS#:17024-55-4
CAS#:17024-55-4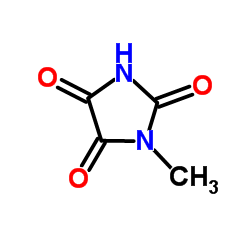 CAS#:3659-97-0
CAS#:3659-97-0 CAS#:37527-30-3
CAS#:37527-30-3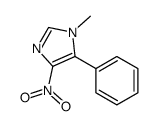 CAS#:111380-10-0
CAS#:111380-10-0![Acetic acid,2-[(1-methyl-4-nitro-1H-imidazol-5-yl)thio]-, ethyl ester structure](https://image.chemsrc.com/caspic/228/6954-33-2.png) CAS#:6954-33-2
CAS#:6954-33-2 CAS#:7464-80-4
CAS#:7464-80-4 CAS#:91089-21-3
CAS#:91089-21-3 CAS#:4531-54-8
CAS#:4531-54-8
