Estriol

Estriol structure
|
Common Name | Estriol | ||
|---|---|---|---|---|
| CAS Number | 50-27-1 | Molecular Weight | 288.381 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 469.0±45.0 °C at 760 mmHg | |
| Molecular Formula | C18H24O3 | Melting Point | 280-282 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 220.8±23.3 °C | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of EstriolEstriol is an antagonist of the G-protein coupled estrogen receptor in estrogen receptor-negative breast cancer cells.Target: Estrogen Receptor/ERRA recent study shows that estrogen (estrone, estradiol, and estriol) inhibits Alzheimer's disease-associated low-order Aβ oligomer formation, and among them, estriol shows the strongest in vitro activity [1]. In mPTEN+/- mice, estriol treatments resulted in a 187.54% gain in the relative ratio of uterine wet weight to body weight; estriol also increases the ratio to 176.88% in wild-type mice [2]. Estriol treatment (20 mg/kg ip), in vivo, sensitizes Kupffer cells to LPS via mechanisms dependent on an increase in CD14 by elevated portal blood endotoxin caused by increased gut permeability in rats; while one-half of the rats given estriol intraperitoneally 24 hours before an injection of a sublethal dose of LPS (5 mg/kg) died within 24 hours [3]. |
| Name | estriol |
|---|---|
| Synonym | More Synonyms |
| Description | Estriol is an antagonist of the G-protein coupled estrogen receptor in estrogen receptor-negative breast cancer cells.Target: Estrogen Receptor/ERRA recent study shows that estrogen (estrone, estradiol, and estriol) inhibits Alzheimer's disease-associated low-order Aβ oligomer formation, and among them, estriol shows the strongest in vitro activity [1]. In mPTEN+/- mice, estriol treatments resulted in a 187.54% gain in the relative ratio of uterine wet weight to body weight; estriol also increases the ratio to 176.88% in wild-type mice [2]. Estriol treatment (20 mg/kg ip), in vivo, sensitizes Kupffer cells to LPS via mechanisms dependent on an increase in CD14 by elevated portal blood endotoxin caused by increased gut permeability in rats; while one-half of the rats given estriol intraperitoneally 24 hours before an injection of a sublethal dose of LPS (5 mg/kg) died within 24 hours [3]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 469.0±45.0 °C at 760 mmHg |
| Melting Point | 280-282 °C(lit.) |
| Molecular Formula | C18H24O3 |
| Molecular Weight | 288.381 |
| Flash Point | 220.8±23.3 °C |
| Exact Mass | 288.172546 |
| PSA | 60.69000 |
| LogP | 2.94 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.624 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H351-H360 |
| Precautionary Statements | P201-P281-P308 + P313 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R60 |
| Safety Phrases | S53-S22-S36/37/39-S45-S36/37 |
| RIDADR | 2811.0 |
| WGK Germany | 3 |
| RTECS | KG8225000 |
| Hazard Class | 6.1 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
|
Development of a novel magnetic molecularly imprinted polymer coating using porous zeolite imidazolate framework-8 coated magnetic iron oxide as carrier for automated solid phase microextraction of estrogens in fish and pork samples.
J. Chromatogr. A. 1365 , 35-44, (2014) A high-performance magnetic molecularly imprinted polymer (MIP) coating using zeolite imidazolate framework-8 coated magnetic iron oxide (Fe3O4@ZIF-8) as a carrier was developed for simultaneous autom... |
|
|
Highly sensitive Fe₃O₄ nanobeads/graphene-based molecularly imprinted electrochemical sensor for 17β-estradiol in water.
Anal. Chim. Acta 884 , 106-13, (2015) A novel molecularly imprinted electrochemical sensor based on Fe3O4 nanobeads immobilized on graphene (Fe3O4-MIP@RGO) has been developed for detecting 17β-estradiol (17β-E2) in water using reversible ... |
|
|
Water-compatible magnetic imprinted nanoparticles served as solid-phase extraction sorbents for selective determination of trace 17beta-estradiol in environmental water samples by liquid chromatography.
J. Chromatogr. A. 1396 , 7-16, (2015) Endocrine disrupting compounds (EDCs) are a potential risk for wildlife and humans for their existence in water. The efficient extraction and clean-up steps are required before detection of low concen... |
| 16α-Estriol |
| OVESTIN |
| TRIOVEX |
| (16a,17b)-Estra-1,3,5(10)-triene-3,16,17-triol |
| 16a-Hydroxyestradiol |
| Tridestrin |
| Oestriol |
| Thulol |
| Ovo-Vinces |
| Colpogyn |
| Orestin |
| MFCD00003691 |
| oekolp |
| Estra-1,3,5(10)-triene-3,16,17-triol, (16α,17β)- |
| Theelol |
| E 3 |
| Estriol |
| 16α,17β-Estriol |
| OE3 |
| (16α,17β)-Oestra-1,3,5(10)-triene-3,16,17-triol |
| 16α-Hydroxyestradiol |
| Aacifemine |
| Holin |
| Ortho-Gynest |
| 16a-Estriol |
| Estriol (JP15/USP) |
| Estratriol |
| Estra-1,3,5(10)-triene-3,16α,17β-triol |
| Gynasan |
| Klimax E |
| trans-Estriol |
| Estra-1,3,5(10)-triene-3,16a,17b-triol |
| (16α,17β)-Estra-1,3,5(10)-triene-3,16,17-triol |
| 3,16α,17β-Trihydroxyestra-1,3,5(10)-triene |
| 3,16a,17b-Trihydroxyestra-1,3,5(10)-triene |
| Estriol [USAN:JAN] |
| 16a,17b-Estriol |
| EINECS 200-022-2 |
| 3,16a,17b-Trihydroxy-D1,3,5-estratriene |
| Hormomed |
| Ovesterin |
| 1,3,5-Estratriene-3b,16a,17b-triol |
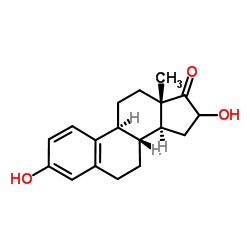 CAS#:566-76-7
CAS#:566-76-7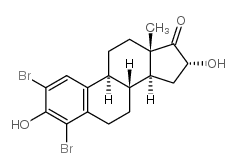 CAS#:79258-14-3
CAS#:79258-14-3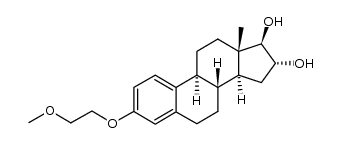 CAS#:123715-88-8
CAS#:123715-88-8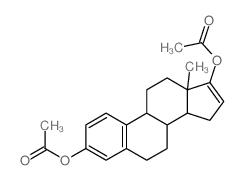 CAS#:20592-42-1
CAS#:20592-42-1 CAS#:1247-71-8
CAS#:1247-71-8 CAS#:69744-63-4
CAS#:69744-63-4 CAS#:53-16-7
CAS#:53-16-7 CAS#:79258-15-4
CAS#:79258-15-4 CAS#:1474-53-9
CAS#:1474-53-9 CAS#:10582-05-5
CAS#:10582-05-5 CAS#:2236-31-9
CAS#:2236-31-9 CAS#:1624-62-0
CAS#:1624-62-0 CAS#:805-26-5
CAS#:805-26-5 CAS#:1852-50-2
CAS#:1852-50-2 CAS#:2479-91-6
CAS#:2479-91-6![1,3,5[10]-ESTRATRIENE-3,16ALPHA,17BETA-TRIHYDROXY 17-GLUCURONIDE structure](https://image.chemsrc.com/caspic/362/7219-89-8.png) CAS#:7219-89-8
CAS#:7219-89-8 CAS#:1169-79-5
CAS#:1169-79-5
