Iberin
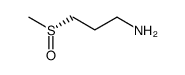
Iberin structure
|
Common Name | Iberin | ||
|---|---|---|---|---|
| CAS Number | 505-44-2 | Molecular Weight | 163.261 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 362.4±25.0 °C at 760 mmHg | |
| Molecular Formula | C5H9NOS2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 173.0±23.2 °C | |
Use of IberinIberin, a sulfoxide analogue of sulforaphane, is a naturally occurring member of isothiocyanate family. It inhibits cell survival with an IC50 of 2.3 μM in HL60 cell. |
| Name | 1-isothiocyanato-3-methylsulfinylpropane |
|---|---|
| Synonym | More Synonyms |
| Description | Iberin, a sulfoxide analogue of sulforaphane, is a naturally occurring member of isothiocyanate family. It inhibits cell survival with an IC50 of 2.3 μM in HL60 cell. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Iberin inhibits the growth of neuroblastoma cells in a dose- and time-dependent manner. The iberin-induced cell cycle arrest in neuroblastoma cells is associated with inhibition of expression of cyclin-dependent kinase Cdk2, Cdk4, and Cdk6 proteins. There is an increase in apoptotic cell death in iberin treated cells as compared with control cells. The iberin-induced apoptosis is found to be associated with activation of caspase-9, caspase-3, and PARP[2]. Iberin inhibits growth of human glioblastoma cells in cell proliferation assays, enhances cytotoxicity, and induces apoptosis by activation of caspase-3 and caspase-9[3]. |
| In Vivo | Iberin is tested in an in vivo foreign-body infection mouse model, and the results show no significantly difference in bacterial clearance between treated and nontreated miced[4]. Iberin increases tissue levels of the phase II detoxification enzymes quinone reductase and glutathione S-transferase in a variety of rat tissues[5]. |
| Cell Assay | Cells are plated at a density of 1×105 cells/well in microtiter plates and treated with different concentrations of iberin (1, 2.5, 10 and 25 μM). Then 20 μL of 5 mg/mL MTT in PBS, is added to each well and allowed to incubate for a further 4 h. After 4 h of incubation, 100 μL of DMSO is added to each well to dissolve the formazan crystals. Absorbance values at 550 nm are measured with a microplate reader[2]. |
| Animal Admin | Rats: Groups of five rats are dosed by oral intubation with the test compounds, as solutions in soybean oil, each day for 5 days. This doses used are 4.0 mg/kg/day for AITC, 5.9 mg/kg/day for iberverin, 6.5 mg/kg/day for iberin, 6.4 mg/kg/day for erucin, 7.1 mg/kg/day for sulforaphane, and 7.2 mg/kg/day for cheirolin. The volume of solution administered is 2 mL/kg in all cases. Ten control rats are dosed with soybean oil alone[5]. Mice: Iberin is diluted in 96% ethanol to a concentration of 32 mg/mL followed by a 40x dilution in 0.9% NaCl. The mice are injected with 0.2 mL of the final solution, corresponding to 8 μg/g of body weight. The placebo group is injected with a 2.4% ethanol solution (96% ethanol–0.9% NaCl) corresponding to the amount of ethanol that the iberin-treated group received. Mice are treated every 12 h from day 2 preinsertion to day 2 postinsertion, and treatment is continued until 12 h before the mice are euthanized[4]. |
| References |
[2]. Jadhav U, et al. Iberin induces cell cycle arrest and apoptosis in human neuroblastoma cells. |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 362.4±25.0 °C at 760 mmHg |
| Molecular Formula | C5H9NOS2 |
| Molecular Weight | 163.261 |
| Flash Point | 173.0±23.2 °C |
| Exact Mass | 163.012558 |
| PSA | 80.73000 |
| LogP | -0.05 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.577 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~50% 
Iberin CAS#:505-44-2 |
| Literature: Moon, Joon-Kwan; Kim, Jun-Ran; Ahn, Young-Joon; Shibamoto, Takayuki Journal of Agricultural and Food Chemistry, 2010 , vol. 58, # 11 p. 6672 - 6677 |
|
~% 
Iberin CAS#:505-44-2 |
| Literature: Karrer et al. Helvetica Chimica Acta, 1950 , vol. 33, p. 1237,1240,1241 |
|
~% 
Iberin CAS#:505-44-2 |
| Literature: Karrer et al. Helvetica Chimica Acta, 1950 , vol. 33, p. 1237,1240,1241 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| sulfpraphane |
| 3-isothiocyanatopropyl methyl sulfoxide |
| METHYLSULFINYLPROPYLISOTHIOCYANATE |
| Propane, 1-isothiocyanato-3-(methylsulfinyl)- |
| 3-Methylsulfinylpropyl isothiocyanate |
| Iberin |
| 1-Isothiocyanato-3-(methylsulfinyl)propane |
| C5H9NOS2 |
| 3-Methylsulphinylpropylisothiocyanate |