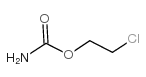
Carbachol

Carbachol structure
|
Common Name | Carbachol | ||
|---|---|---|---|---|
| CAS Number | 51-83-2 | Molecular Weight | 182.648 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C6H15ClN2O2 | Melting Point | 200-204 ºC | |
| MSDS | Chinese USA | Flash Point | 90°C(lit.) | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of CarbacholCarbamoylcholine chloride is used to study responses mediated by nAChR and mAChR, including smooth muscle contraction, gut motility, and neuronal signaling.IC50 value: 10 to 10,000 nM (Ki)Target: nAChR, mAChRCarbamoylcholine is an analog of acetylcholine that activates acetylcholine receptors (AChR). Carbamoylcholine is an agonist of both nicotinic (nAChR) and muscarinic (mAChR) receptors, with reported Ki values ranging from 10 to 10,000 nM for different receptors and different preparations. |
| Name | Carbachol |
|---|---|
| Synonym | More Synonyms |
| Description | Carbamoylcholine chloride is used to study responses mediated by nAChR and mAChR, including smooth muscle contraction, gut motility, and neuronal signaling.IC50 value: 10 to 10,000 nM (Ki)Target: nAChR, mAChRCarbamoylcholine is an analog of acetylcholine that activates acetylcholine receptors (AChR). Carbamoylcholine is an agonist of both nicotinic (nAChR) and muscarinic (mAChR) receptors, with reported Ki values ranging from 10 to 10,000 nM for different receptors and different preparations. |
|---|---|
| Related Catalog |
| Melting Point | 200-204 ºC |
|---|---|
| Molecular Formula | C6H15ClN2O2 |
| Molecular Weight | 182.648 |
| Flash Point | 90°C(lit.) |
| Exact Mass | 182.082199 |
| PSA | 52.32000 |
| Appearance of Characters | crystalline | white |
| Water Solubility | 1.0 G/ML |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 |
| Precautionary Statements | P264-P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R25;R36/37/38 |
| Safety Phrases | S45-S36/37/39-S28A-S26 |
| RIDADR | UN 2811 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | GA0875000 |
| Packaging Group | II |
| Hazard Class | 6.1 |
| HS Code | 2924199090 |
|
~% 
Carbachol CAS#:51-83-2 |
| Literature: Journal of the Chemical Society, , p. 179 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
A biosensor to monitor dynamic regulation and function of tumour suppressor PTEN in living cells.
Nat. Commun. 5 , 4431, (2014) Tumour suppressor PTEN is a phosphatase that negatively regulates the PI3K/AKT pathway. The ability to directly monitor PTEN conformation and function in a rapid, sensitive manner is a key step toward... |
|
|
Taurolithocholic acid promotes intrahepatic cholangiocarcinoma cell growth via muscarinic acetylcholine receptor and EGFR/ERK1/2 signaling pathway.
Int. J. Oncol. 46 , 2317-26, (2015) Cholangiocarcinoma (CCA) is a malignant cancer of the biliary tract and its occurrence is associated with chronic cholestasis which causes an elevation of bile acids in the liver and bile duct. The pr... |
|
|
Involvement of the Tyr kinase/JNK pathway in carbachol-induced bronchial smooth muscle contraction in the rat.
Anesthesiology 118(5) , 1076-85, (2013) Tyrosine (Tyr) kinases and mitogen-activated protein kinases have been thought to participate in the contractile response in various smooth muscles. The aim of the current study was to investigate the... |
| ethanaminium, 2-[(aminocarbonyl)oxy]-N,N,N-trimethyl-, chloride |
| EINECS 200-127-3 |
| 2-carbamoyloxyethyl(trimethyl)azanium,chloride |
| Ethanaminium, 2-[(aminocarbonyl)oxy]-N,N,N-trimethyl-, chloride (1:1) |
| Ethanaminium, 2-((aminocarbonyl)oxy)-N,N,N-trimethyl-, chloride |
| Carbamoylcholine chloride |
| (2-Hydroxyethyl)trimethylammonium chloride carbamate Carbachol Carbamylcholine chloride |
| Carbachol,Carbamylcholine chloride |
| MFCD00012011 |
| Carbamylcholine Chloride |
| (2-Hydroxyethyl)trimethylammonium chloride carbamate,Carbachol,Carbamoylcholine chloride |
| 2-(Carbamoyloxy)-N,N,N-trimethylethanaminium chloride |
| Carbamoylcholine (chloride) |