Acipimox
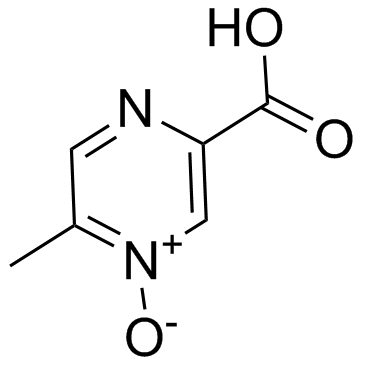
Acipimox structure
|
Common Name | Acipimox | ||
|---|---|---|---|---|
| CAS Number | 51037-30-0 | Molecular Weight | 154.123 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 539.0±45.0 °C at 760 mmHg | |
| Molecular Formula | C6H6N2O3 | Melting Point | 177-180 °C | |
| MSDS | Chinese USA | Flash Point | 279.8±28.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of AcipimoxAcipimox is a niacin derivative used as a hypolipidemic agent.Target: Acipimox is a niacin derivative used as a hypolipidemic agent. It is used in low doses and may have less marked adverse effects, although it is unclear whether the recommended dose is as effective as are standard doses of nicotinic acid. Acipimox inhibits the production of triglycerides by the liver and the secretion of VLDL, which leads indirectly to a modest reduction in LDL and increase in HDL. Long-term administration is associated with reduced mortality, but unwanted effects limit its clinical use. Adverse effects include flushing (associated with Prostaglandin D2), palpitations, and GI disturbances. Flushing can be reduced by taking aspirin 20-30 min before taking Acipimox. High doses can cause disorders of liver function, impair glucose tolerance and precipitate gout. From Wikipedia. |
| Name | 5-methyl-4-oxidopyrazin-4-ium-2-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Acipimox is a niacin derivative used as a hypolipidemic agent.Target: Acipimox is a niacin derivative used as a hypolipidemic agent. It is used in low doses and may have less marked adverse effects, although it is unclear whether the recommended dose is as effective as are standard doses of nicotinic acid. Acipimox inhibits the production of triglycerides by the liver and the secretion of VLDL, which leads indirectly to a modest reduction in LDL and increase in HDL. Long-term administration is associated with reduced mortality, but unwanted effects limit its clinical use. Adverse effects include flushing (associated with Prostaglandin D2), palpitations, and GI disturbances. Flushing can be reduced by taking aspirin 20-30 min before taking Acipimox. High doses can cause disorders of liver function, impair glucose tolerance and precipitate gout. From Wikipedia. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 539.0±45.0 °C at 760 mmHg |
| Melting Point | 177-180 °C |
| Molecular Formula | C6H6N2O3 |
| Molecular Weight | 154.123 |
| Flash Point | 279.8±28.7 °C |
| Exact Mass | 154.037842 |
| PSA | 75.65000 |
| LogP | -1.38 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.608 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36 |
| Safety Phrases | S26 |
| RIDADR | NONH for all modes of transport |
| RTECS | UQ2453000 |
| HS Code | 2942000000 |
|
~% 
Acipimox CAS#:51037-30-0 |
| Literature: Carlo Erba S.p.A. Patent: US4002750 A1, 1977 ; |
|
~% 
Acipimox CAS#:51037-30-0 |
| Literature: Carlo Erba S.p.A. Patent: US4002750 A1, 1977 ; |
|
~% 
Acipimox CAS#:51037-30-0 |
| Literature: Montedison S.p.A. Patent: US4866178 A1, 1989 ; |
|
~% 
Acipimox CAS#:51037-30-0 |
| Literature: Ambrogi; Cozzi; Sanjust; et al. European Journal of Medicinal Chemistry, 1980 , vol. 15, # 2 p. 157 - 163 |
|
~% 
Acipimox CAS#:51037-30-0 |
| Literature: Ambrogi; Cozzi; Sanjust; et al. European Journal of Medicinal Chemistry, 1980 , vol. 15, # 2 p. 157 - 163 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Kinetics and utilization of lipid sources during acute exercise and acipimox.
Am. J. Physiol. Endocrinol. Metab. 307(2) , E199-208, (2014) Overweight is associated with abnormalities of lipid metabolism, many of which are reversed by exercise. We investigated the impact of experimental antilipolysis and acute exercise on lipid kinetics a... |
|
|
The lowering of hepatic fatty acid uptake improves liver function and insulin sensitivity without affecting hepatic fat content in humans.
Am. J. Physiol. Endocrinol. Metab. 295(2) , E413-9, (2008) Lipolysis may regulate liver free fatty acid (FFA) uptake and triglyceride accumulation; both are potential causes of insulin resistance and liver damage. We evaluated whether 1) systemic FFA release ... |
|
|
ECG-triggered 18F-fluorodeoxyglucose positron emission tomography imaging of the rat heart is dramatically enhanced by acipimox.
Eur. J. Nucl. Med. Mol. Imaging 37(9) , 1745-50, (2010) 18F-Fluorodeoxyglucose (FDG) imaging, provided by current positron emission tomography (PET) systems dedicated to small animals,might provide a precise functional assessment of the left ventricle (LV)... |
| 5-methyl-2-pyrazinoic-4-oxide |
| 2-Pyrazinecarboxylic acid, 5-methyl-, 4-oxide |
| 2-Carboxy-5-methylpyrazine 4-oxide,5-Methylpyrazinecarboxylic acid 4-oxide |
| 5-Carboxy-2-methylpyrazine 1-oxide |
| 5-methyl-4-oxy-pyrazine-2-carboxylic acid |
| 5-methylpyrazine-2-carboxylic acid 4-oxide |
| 2-carboxy-5-methylpyrazine 4-oxide |
| Acipimox |
| K-9321 |
| Olbetam |
| 5-Methylpyrazinecarboxylic acid 4-oxide |
| 5-Methyl-2-pyrazinecarboxylic acid 4-oxide |
| 2-methyl-5-pyrazinecarboxylic acid 1-oxide |
| Olbemox |
| EINECS 256-928-3 |
| T6N DNJ AO B1 EVQ |
| MFCD00865757 |

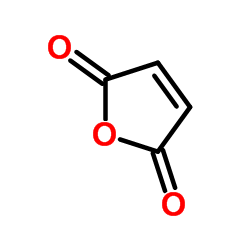
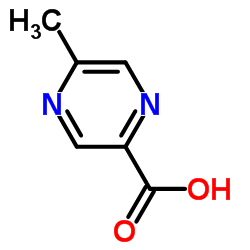
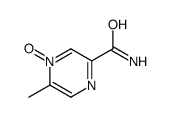

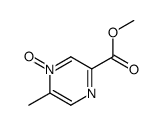 CAS#:74416-38-9
CAS#:74416-38-9 CAS#:83056-59-1
CAS#:83056-59-1
