3-Methyladenine

3-Methyladenine structure
|
Common Name | 3-Methyladenine | ||
|---|---|---|---|---|
| CAS Number | 5142-23-4 | Molecular Weight | 149.153 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 240.1±50.0 °C at 760 mmHg | |
| Molecular Formula | C6H7N5 | Melting Point | -300ºC (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 99.0±30.1 °C | |
Use of 3-Methyladenine3-Methyladenine is a PI3K inhibitor. 3-Methyladenine is a widely used inhibitor of autophagy via its inhibitory effect on class III PI3K. |
| Name | 3-methyladenine |
|---|---|
| Synonym | More Synonyms |
| Description | 3-Methyladenine is a PI3K inhibitor. 3-Methyladenine is a widely used inhibitor of autophagy via its inhibitory effect on class III PI3K. |
|---|---|
| Related Catalog | |
| Target |
PtdIns3Kγ:60 μM (IC50, Cell Assay) Vps34:25 μM (IC50, Cell Assay) Human Endogenous Metabolite Autophagy Mitophagy |
| In Vitro | 3-Methyladenine shows slight preference to binds to Vps34 in vitro, with an IC50 of 25 µM for Vps34 as compared with 60 µM for PtdIns3Kγ, and 3-Methyladenine (10 mM) can inhibit all PtdIns3Ks[1]. 3-Methyladenine (3-MA, 5 mM) suppresses autophagy in HeLa cells under both glucose-free conditions and normal conditions. 3-Methyladenine (2.5, 5 or 10 mM) induces caspase-dependent cell death in HeLa cells, and the death occurs independently of the inhibition of autophagy. Moreover, 3-Methyladenine (3-MA, 1 mM) significantly shortens the duration of nocodazole-induced-prometaphase arrest[2]. |
| In Vivo | 3-Methyladenine (1.5 mg/100 g, i.p.) treatment alleviates sodium taurocholate-induced severe acute pancreatitis (SAP) in rats at both 12 and 24 h. 3-Methyladenine inhibits autophagy of pancreatic acinar cells in sodium taurocholate-tnduced SAP. 3-Methyladenine also shows inhibitory effects on PI3K/Akt signaling pathway and NF-κB signaling pathway in sodium taurocholate-tnduced SAP[3]. |
| Cell Assay | Cell viability is determined by a trypan blue exclusion assay. Cells are cultured in the medium with 3-Methyladenine. Both adherent and floating cells are collected and suspended in phosphate buffered saline (PBS, pH 7.4) at a final density of 1-2×106/mL. An equal volume of 0.4% trypan blue solution (w/v, in PBS) is added to the cell suspension and mixed thoroughly. After incubation at room temperature for 3 min, cell counting is performed using a hemacytometer. |
| Animal Admin | All rats are fasted for 12 h with free access to water prior to operation. After anesthesia by intraperitoneal (i.p.) injection of 2% sodium pentobarbital (0.25 mL/100 g), they are laid and fixed on the table, routinely shaven, disinfected, and draped. The rat SAP model is induced by 0.1 mL/min speed uniformly retrograde infusion of a freshly prepared 3.5% sodium taurocholate solution (0.1 mL/100 g) into the biliopancreatic duct after laparotomy. Equivalent volume of normal saline solution is substituted for 3.5% sodium taurocholate solution in the sham-operation (SO) control group. The incision is closed with a continuous 3-0-silk suture, and 2 mL/100 g of saline is injected into the back subcutaneously to compensate for the fluid loss. 180 rats are randomly divided into four groups: (1) Acanthopanax treatment group (Aca group, n = 45) where the rats are injected with 0.2% Acanthopanax injection at a dose of 3.5 mg/100 g 3 h after successful modeling via the vena caudalis once, knowing that this dosage is effective; (2) 3-Methyladenine treatment group (3-methyladenine group, n = 45) where the rats are injected with 100 nmol/μL 3-methyladenine solution at a dose of 1.5 mg/100 g 3 h after successful modeling via the intraperitoneal route once, knowing that this dosage is effective; (3) SAP model group (SAP group, n = 45) where these rats receive an equivalent volume of the normal saline instead of Acanthopanax injection 3 h after successful modeling via the vena caudalis once; (4) SO group (control, n = 45) where these rats receive an equivalent volume of the normal saline instead of Acanthopanax injection 3 h after successful sham-operation via the vena caudalis once. The 45 animals in each of the four groups are equally randomized into 3, 12, and 24 h subgroups for postoperative observations[3]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 240.1±50.0 °C at 760 mmHg |
| Melting Point | -300ºC (dec.)(lit.) |
| Molecular Formula | C6H7N5 |
| Molecular Weight | 149.153 |
| Flash Point | 99.0±30.1 °C |
| Exact Mass | 149.070145 |
| PSA | 69.62000 |
| LogP | -2.36 |
| Appearance of Characters | Powder | White |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.807 |
| Storage condition | Store at +4°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Neuroprotective effect of modified Chungsimyeolda-tang, a traditional Korean herbal formula, via autophagy induction in models of Parkinson's disease.
J. Ethnopharmacol. 159 , 93-101, (2014) Previous studies in our laboratory revealed the neuroprotective effect of modified Yeoldahanso-tang (MYH) in models of Parkinson׳s disease (PD). In this study, we investigated another traditional Kore... |
|
|
Inhibition of autophagy suppresses sertraline-mediated primary ciliogenesis in retinal pigment epithelium cells.
PLoS ONE 10(2) , e0118190, (2015) Primary cilia are conserved cellular organelles that regulate diverse signaling pathways. Autophagy is a complex process of cellular degradation and recycling of cytoplasmic proteins and organelles, a... |
|
|
A novel functional interplay between Progesterone Receptor-B and PTEN, via AKT, modulates autophagy in breast cancer cells.
J. Cell. Mol. Med. 18(11) , 2252-65, (2014) The tumour suppressor activity of the phosphatase and tensin homologue on chromosome 10 (PTEN) is subject of intense investigative efforts, although limited information on its regulation in breast can... |
| 3-Methyl-3H-purin-6-amine |
| EINECS 225-908-6 |
| methyladenine |
| N(3)-methyladenine |
| 3-methyl-3H-adenine |
| 3-MA,6-Amino-3-methylpurine |
| 3-methylpurin-6-amine |
| MFCD00010531 |
| 3H-Purin-6-amine, 3-methyl- |
| 6-Amino-3-methylpurine |
| 3-Methyladenine |
| 3-MA |
 CAS#:76227-27-5
CAS#:76227-27-5 CAS#:125661-70-3
CAS#:125661-70-3 CAS#:3506-01-2
CAS#:3506-01-2 CAS#:76227-20-8
CAS#:76227-20-8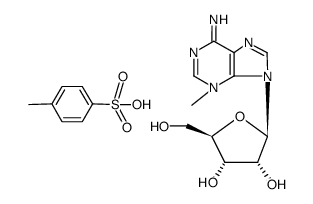 CAS#:72055-63-1
CAS#:72055-63-1 CAS#:21149-25-7
CAS#:21149-25-7 CAS#:66224-66-6
CAS#:66224-66-6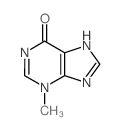 CAS#:1006-11-7
CAS#:1006-11-7
