Shikonine
Modify Date: 2024-01-02 16:54:11
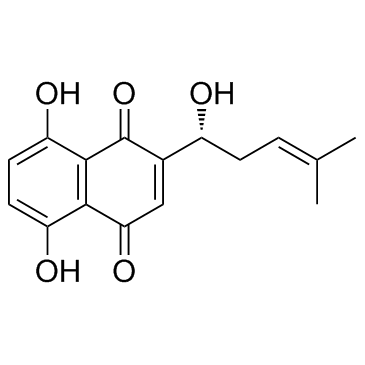
Shikonine structure
|
Common Name | Shikonine | ||
|---|---|---|---|---|
| CAS Number | 517-89-5 | Molecular Weight | 288.295 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 567.4±50.0 °C at 760 mmHg | |
| Molecular Formula | C16H16O5 | Melting Point | 147ºC | |
| MSDS | N/A | Flash Point | 311.0±26.6 °C | |
Use of ShikonineShikonin is a major component of a Chinese herbal medicine named zicao. Shikonin has shown various biological activities, including inhibition of TNF-α, NF-κB, HIV-1. |
| Name | (R)-5,8-Dihydroxy-2-(1-hydroxy-4-methylpent-3-en-1-yl)naphthalene-1,4-dione |
|---|---|
| Synonym | More Synonyms |
| Description | Shikonin is a major component of a Chinese herbal medicine named zicao. Shikonin has shown various biological activities, including inhibition of TNF-α, NF-κB, HIV-1. |
|---|---|
| Related Catalog | |
| Target |
NF-κB TNF-α TMEM16A chloride channel:6.5 μM (IC50) PKM2 |
| In Vitro | Shikonin is an inhibitor of TMEM16A chloride channel with an IC50 of 6.5 μM[1]. Shikonin is also a specific inhibitor of PKM2[2] and can also inhibit tumor necrosis factor-α (TNF-α) and prevent activation of nuclear factor-κB (NF-κB) pathway. Shikonin at concentrations higher than 50 μM significantly inhibits ormal human keratinocytes (NHKs) viability, compare with that of control (P<0.05). Pretreatment with Shikonin for 2 h attenuates TNF-α-induced NF-κB p65 nuclear translocation[3]. Treatments of Shikonin at 5 and 7.5 μM significantly inhibit the cell viability starting from 12 h and the inhibitory effects are presented in time-dependent patterns compare with the 0 h group in both cell lines. It is found that 5 μM Shikonin displays greater inhibition compare to 2.5 μM at the time points from 24 to 48 h. The invasiveness of U87 and U251 cells is significantly attenuated when treated with Shikonin at 2.5, 5, and 7.5 μM compare with the control group at 24 and 48 h (p<0.01)[4]. |
| In Vivo | Shikonin significantly inhibits the increase in IL-1β and TNF-α expression levels in the rat model of osteoarthritis, compare with those in the osteoarthritis group (P<0.01). The NF-κB protein expression level is significantly suppressed by Shikonin in the rat model of osteoarthritis, compare with that in the osteoarthritis group (P<0.01). The induction of the iNOS level is suppressed by treatment with Shikonin in the rat model of osteoarthritis, compare with that in the osteoarthritis group (P<0.01). The administration of Shikonin markedly weakens the up-regulation of COX-2 protein expression in the rat model of osteoarthritis, as compare with that in the osteoarthritis group (P<0.01). The elevation of caspase-3 activity is significantly reduced by Shikonin treatment in the rat model of osteoarthritis, compare with that in the osteoarthritis group (P<0.01). The downregulation of Akt phosphorylation is also significantly recovered by treatment with Shikonin in the rat model of osteoarthritis, compare with that in the osteoarthritis group (P<0.01)[5]. |
| Cell Assay | U87 and U251 cells are seeded into 96-well plates at a density of 1×104 cells per well in standard DMEM and incubated for 24 h under standard conditions (37°C and 5% CO2). Then the medium is replaced with either blank, serum-free DMEM or DMEM containing Shikonin at concentrations of 2.5, 5, and 7.5 μM. The total volume in each well is 200 μL. Finally, the plates are shaken softly and the optical density is recorded at 570 nm (OD570) using a plate reader. At least three independent experiments are performed[4]. |
| Animal Admin | Healthy male Sprague-Dawley rats (n=30; 8 to 10-weeks old, 250 to 300 g) are used in this study. Rats are randomly assigned to three groups: Sham-operated group (n=10), osteoarthritis model group (n=10) and Shikonin-treated group (n=10). In the sham-operated group, the right knee joint of the anesthetized rat is only exposed under sterile conditions, and the rats are treated with 0.1 ml/100 g physiological saline (i.p.). In the osteoarthritis model group, osteoarthritis model rats were treated with 0.1 ml/100 g physiological saline (i.p.). In the Shikonin-treated group, osteoarthritis model rats are treated with 10 mg/kg Shikonin (i.p.) once daily for 4 days after osteoarthritis modeling[5]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 567.4±50.0 °C at 760 mmHg |
| Melting Point | 147ºC |
| Molecular Formula | C16H16O5 |
| Molecular Weight | 288.295 |
| Flash Point | 311.0±26.6 °C |
| Exact Mass | 288.099762 |
| PSA | 94.83000 |
| LogP | 4.35 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.642 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn |
|---|---|
| Risk Phrases | R20/21/22 |
| Safety Phrases | S26-S36/37/39 |
| HS Code | 2914690090 |
| Precursor 7 | |
|---|---|
| DownStream 2 | |
| HS Code | 2914690090 |
|---|---|
| Summary | 2914690090 other quinones。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
| 5,8-Dihydroxy-2-[(1R)-1-hydroxy-4-methylpent-3-en-1-yl]-1,4-naphthoquinone |
| 1,4-Naphthalenedione, 5,8-dihydroxy-2-[(1R)-1-hydroxy-4-methyl-3-penten-1-yl]- |
| 1,4-Naphthalenedione, 5,8-dihydroxy-2-[(1R)-1-hydroxy-4-methyl-3-pentenyl]- |
| MFCD00075680 |
| Shikonin |
| 5,8-Dihydroxy-2-[(1R)-1-hydroxy-4-methyl-3-penten-1-yl]-1,4-naphthoquinone |
| (+)-Alkannin |
| 5,8-Dihydroxy-2-[(1R)-1-hydroxy-4-methyl-pent-3-enyl]naphthalene-1,4-dione |
![(R)-4-methyl-1-(naphtho[1,8-de:4,5-d'e']bis([1,3]dioxine)-4-yl)pent-3-en-1-ol Structure](https://image.chemsrc.com/caspic/311/206996-08-9.png) CAS#:206996-08-9
CAS#:206996-08-9![4-BROMONAPHTHO[1,8-DE:4,5-D'E']BIS([1,3]DIOXINE) Structure](https://image.chemsrc.com/caspic/244/88051-30-3.png) CAS#:88051-30-3
CAS#:88051-30-3![NAPHTHO[1,8-DE:4,5-D'E']BIS([1,3]DIOXINE) Structure](https://image.chemsrc.com/caspic/147/88051-28-9.png) CAS#:88051-28-9
CAS#:88051-28-9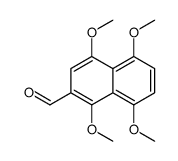 CAS#:88818-28-4
CAS#:88818-28-4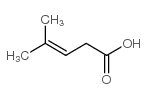 CAS#:504-85-8
CAS#:504-85-8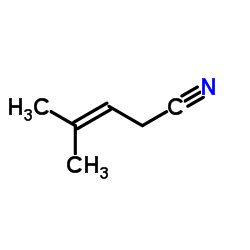 CAS#:4786-23-6
CAS#:4786-23-6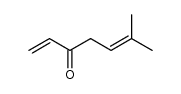 CAS#:33698-69-0
CAS#:33698-69-0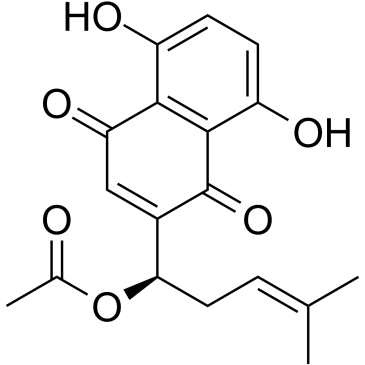 CAS#:24502-78-1
CAS#:24502-78-1 CAS#:517-90-8
CAS#:517-90-8
