Cycloleucine
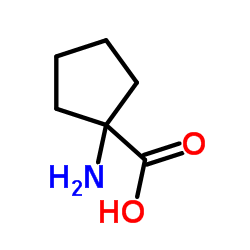
Cycloleucine structure
|
Common Name | Cycloleucine | ||
|---|---|---|---|---|
| CAS Number | 52-52-8 | Molecular Weight | 129.157 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 256.1±23.0 °C at 760 mmHg | |
| Molecular Formula | C6H11NO2 | Melting Point | 320 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 108.7±22.6 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of CycloleucineCycloleucine is a specific inhibitor of S-adenosyl-methionine mediated methylation. Cycloleucine is antagonist of NMDA receptor associated glycine receptor, with a Ki of 600 μM. Cycloleucine is also a competitive inhibitor of ATP: L-methionine-S-adenosyl transferase in vitro. Cycloleucine has anxiolytic and cytostatic effects[1][2][3][4]. |
| Name | 1-aminocyclopentanecarboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Cycloleucine is a specific inhibitor of S-adenosyl-methionine mediated methylation. Cycloleucine is antagonist of NMDA receptor associated glycine receptor, with a Ki of 600 μM. Cycloleucine is also a competitive inhibitor of ATP: L-methionine-S-adenosyl transferase in vitro. Cycloleucine has anxiolytic and cytostatic effects[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 600 μM (NMDA)[1][2] |
| In Vitro | Cycloleucine (4-40 mM; 3 h) blocks internal methylation of viral RNA in B77 transformed chick embryo fibroblasts[5]. Cycloleucine (40 mM; 24 h) blocks the formation of both m6A and the penultimate Gm in B77 38S RNA subunits by greater than 90%[5]. Cytostatic (10 µg/mL) inhibits the viability human KB and mouse L1210s leukemia cell lines[5]. |
| In Vivo | Cycloleucine (0.5-4 µg; intracerebroventrical injection) increases time spent in open arms, open arm entries, and extreme arrivals in rats[3]. Animal Model: Male rats bilaterally cannulated into the nucleus accumbens septi (NAS)[3] Dosage: 1 µL of 0.5, 1.0, 2.0, 4 µg/µL Administration: Intracerebroventrical injection Result: Increased time spent in the open arms and extreme arrivals at all doses. Increased open arm entries at the dose of 4 μg. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 256.1±23.0 °C at 760 mmHg |
| Melting Point | 320 °C (dec.)(lit.) |
| Molecular Formula | C6H11NO2 |
| Molecular Weight | 129.157 |
| Flash Point | 108.7±22.6 °C |
| Exact Mass | 129.078979 |
| PSA | 63.32000 |
| LogP | -0.05 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.522 |
| Water Solubility | 5 g/100 mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | GY2625000 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2922499990 |
|
~75% 
Cycloleucine CAS#:52-52-8 |
| Literature: Tsang, Joseph W.; Schmied, Bernhard; Nyfeler, Rolf; Goodman, Murray Journal of Medicinal Chemistry, 1984 , vol. 27, # 12 p. 1663 - 1668 |
|
~% 
Cycloleucine CAS#:52-52-8 |
| Literature: Kovachev; Ivanov; Buyukliev; Konstantinov; Karaivanova Pharmazie, 1996 , vol. 51, # 1 p. 25 - 27 |
|
~% 
Cycloleucine CAS#:52-52-8 |
| Literature: Neelakantan; Hartung Journal of Organic Chemistry, 1958 , vol. 23, p. 964,967 |
|
~% 
Cycloleucine CAS#:52-52-8 |
| Literature: Neelakantan; Hartung Journal of Organic Chemistry, 1958 , vol. 23, p. 964,967 |
|
~% 
Cycloleucine CAS#:52-52-8 |
| Literature: Neelakantan; Hartung Journal of Organic Chemistry, 1958 , vol. 23, p. 964,967 |
|
~% 
Cycloleucine CAS#:52-52-8 |
| Literature: O'Donnell, Martin J.; Bruder, William A.; Eckrich, Thomas M.; Shullenberger, Daniel F.; Staten, Gilbert S. Synthesis, 1984 , # 2 p. 127 - 128 |
|
~% 
Cycloleucine CAS#:52-52-8 |
| Literature: Sudo,R.; Ichihara,S. Bulletin of the Chemical Society of Japan, 1963 , vol. 36, p. 34 - 37 |
|
~% 
Cycloleucine CAS#:52-52-8 |
| Literature: Zelinsky; Annenkow; Kulikow Zhurnal Russkago Fiziko-Khimicheskago Obshchestva, 1911 , vol. 43, p. 1095 Hoppe-Seyler's Zeitschrift fuer Physiologische Chemie, 1911 , vol. 73, p. 463 Full Text Show Details Zelinsky; Stadnikow Hoppe-Seyler's Zeitschrift fuer Physiologische Chemie, 1911 , vol. 75, p. 350 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2922499990 |
|---|---|
| Summary | HS:2922499990 other amino-acids, other than those containing more than one kind of oxygen function, and their esters; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:6.5% General tariff:30.0% |
|
Quantitative structure-activity relationship and complex network approach to monoamine oxidase A and B inhibitors.
J. Med. Chem. 51 , 6740-51, (2008) The work provides a new model for the prediction of the MAO-A and -B inhibitor activity by the use of combined complex networks and QSAR methodologies. On the basis of the obtained model, we prepared ... |
|
|
In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase.
J. Neurosci. 33(19) , 8411-22, (2013) Calcium-dependent release of vasoactive gliotransmitters is widely assumed to trigger vasodilation associated with rapid increases in neuronal activity. Inconsistent with this hypothesis, intact stimu... |
|
|
Drug design, in vitro pharmacology, and structure-activity relationships of 3-acylamino-2-aminopropionic acid derivatives, a novel class of partial agonists at the glycine site on the N-methyl-D-aspartate (NMDA) receptor complex.
J. Med. Chem. 52 , 5093-107, (2009) Retaining agonistic activity at the glycine coagonist site of the NMDA receptor in molecules derived from glycine or d-serine has proven to be difficult because in the vicinity of the alpha-amino acid... |
| 1-Aminocyclopentanecarboxylicacid |
| 1-aminocyclopentane-1-carboxylic acid |
| 1-Aminocyclopropane-1-carboxylic acid |
| EINECS 200-144-6 |
| 1-amino-1-carboxylic cyclopentane |
| 1-Amino-1-cyclopentanecarboxylic acid |
| ACPC |
| Cyclopentanecarboxylic acid, 1-amino- |
| MFCD00001381 |
| Cycloleucine |
| 1-amino-1-carboxycyclopentane |
| Cyclolencine |
| 1-Aminocyclopentanecarboxylic acid |
| 1-Amino-1-cyclopentanecarboxylate |
| 1-aminocyclopentane-1-carboxylicacid |
![1,3-Diazaspiro[4.4]nonane-2,4-dione structure](https://image.chemsrc.com/caspic/354/699-51-4.png)




 CAS#:3400-45-1
CAS#:3400-45-1 CAS#:35264-09-6
CAS#:35264-09-6![7,14-diazadispiro[4.2.48.25]tetradecane-6,13-dione structure](https://image.chemsrc.com/caspic/320/14855-33-5.png) CAS#:14855-33-5
CAS#:14855-33-5![6,9-DIAZA-SPIRO[4.5]DECANEDIHYDROCHLORIDE structure](https://image.chemsrc.com/caspic/309/145122-55-0.png) CAS#:145122-55-0
CAS#:145122-55-0![N-[(2'-Cyano[1,1'-biphenyl]-4-yl)methyl]-1-[(1-oxopentyl)amino]cyclopentanecarboxamide structure](https://image.chemsrc.com/caspic/247/141745-71-3.png) CAS#:141745-71-3
CAS#:141745-71-3 CAS#:17193-28-1
CAS#:17193-28-1 CAS#:22649-36-1
CAS#:22649-36-1 CAS#:22649-37-2
CAS#:22649-37-2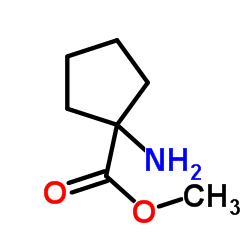 CAS#:78388-61-1
CAS#:78388-61-1
