Fraxin
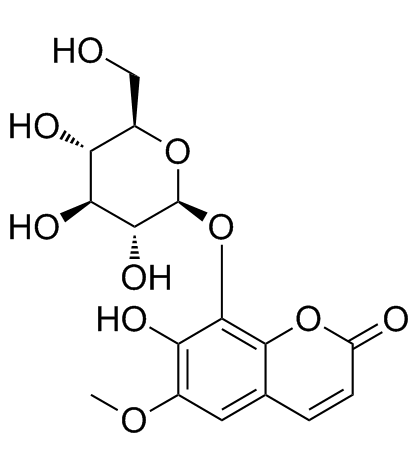
Fraxin structure
|
Common Name | Fraxin | ||
|---|---|---|---|---|
| CAS Number | 524-30-1 | Molecular Weight | 370.308 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 722.2±60.0 °C at 760 mmHg | |
| Molecular Formula | C16H18O10 | Melting Point | 205-208ºC | |
| MSDS | Chinese USA | Flash Point | 267.1±26.4 °C | |
Use of FraxinFraxin isolated from Acer tegmentosum, F. ornus or A. hippocastanum, is a glucoside of fraxetin and reported to exert potent anti-oxidative stress action[1], anti-inflammatory and antimetastatic properties. Fraxin shows its antioxidative effect through inhibition of cyclo AMP phosphodiesterase enzyme[2]. |
| Name | fraxin |
|---|---|
| Synonym | More Synonyms |
| Description | Fraxin isolated from Acer tegmentosum, F. ornus or A. hippocastanum, is a glucoside of fraxetin and reported to exert potent anti-oxidative stress action[1], anti-inflammatory and antimetastatic properties. Fraxin shows its antioxidative effect through inhibition of cyclo AMP phosphodiesterase enzyme[2]. |
|---|---|
| Related Catalog | |
| Target |
Cyclo AMP phosphodiesterase enzyme[2]. |
| In Vitro | Fraxin (100 μM) is non-cytotoxic on Hep G2 cells. Fraxin at non-cytotoxic concentrations significantly decreases the t-BHP-induced ROS generation in a dose-dependent manner[1]. Fraxin (0.5 mM) shows free radical scavenging effect at high concentration and cell protective effect against H2O2-mediated oxidative stress[2]. |
| In Vivo | Fraxin (50 mg/kg, p.o.) significantly blocks the CCl4-induced elevation of ALT and AST. Fraxin (10 and 50 mg/kg, p.o.) significantly reduces the GSSG levels (1.7±0.3 and 1.5±0.2 nM/g liver, respectively) compared with the GSSG levels of the CCl4-treated group[1]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 722.2±60.0 °C at 760 mmHg |
| Melting Point | 205-208ºC |
| Molecular Formula | C16H18O10 |
| Molecular Weight | 370.308 |
| Flash Point | 267.1±26.4 °C |
| Exact Mass | 370.089996 |
| PSA | 159.05000 |
| LogP | -1.93 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.664 |
| Storage condition | 2-8°C |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | DJ3091000 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
|
Screening for novel quorum-sensing inhibitors to interfere with the formation of Pseudomonas aeruginosa biofilm.
J. Med. Microbiol. 60(Pt 12) , 1827-34, (2011) The objective of this study was to screen for novel quorum-sensing inhibitors (QSIs) from traditional Chinese medicines (TCMs) that inhibit bacterial biofilm formation. Six of 46 active components fou... |
|
|
[Studies on chemical constituents from stem barks of Fraxinus paxiana].
Zhongguo Zhong Yao Za Zhi 33(16) , 1990-3, (2008) To investigate the chemical constituents of Fraxinus paxiana.The chemical constituents were isolated and purified by chromatographic techniques and the structures of the compounds were identified with... |
|
|
[Coumarins from branch of Fraxinus sieboldiana and their antioxidative activity].
Zhongguo Zhong Yao Za Zhi 33(14) , 1708-10, (2008) To investigate the chemical constituents from the branch of Fraxinus sieboldiana, and evaluate their antioxidative activity.The chemical constituents were isolated and purified by chromatographic tech... |
| 7,8-Dihydroxy-6-methoxycoumarin-8-b-D-glucoside |
| 7-Hydroxy-6-methoxy-2-oxo-2H-chromen-8-yl β-D-glucopyranoside |
| 8-(Glucosyloxy)-6-methoxyumbelliferone |
| UNII-V7M270Y072 |
| 2H-1-Benzopyran-2-one, 8-(β-D-glucopyranosyloxy)-7-hydroxy-6-methoxy- |
| 7-Hydroxy-6-méthoxy-8-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxyméthyl)tétrahydro-2H-pyran-2-yl]oxy}-2H-chromén-2-one |
| 7-Hydroxy-6-methoxy-8-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}-2H-chromen-2-on |
| 8-(β-D-Glucopyranosyloxy)-7-hydroxy-6-methoxy-2H-1-benzopyran-2-one |
| Fraxin |
| 8-(b-D-Glucopyranosyloxy)-7-hydroxy-6-methoxy-2H-1-benzopyran-2-one |
| Fraxoside |
| Fraxetin-8-O-glucoside |
| Fraxetin-8-glucoside |
| Paviin |
| 7-Hydroxy-6-methoxy-2-oxo-2H-chromen-8-yl-β-D-glucopyranoside |
| Fraxetol 8-glucoside |
| 7-Hydroxy-6-methoxy-8-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}-2H-chromen-2-one |
 CAS#:574-84-5
CAS#:574-84-5
