Boc-D-Trp-OH
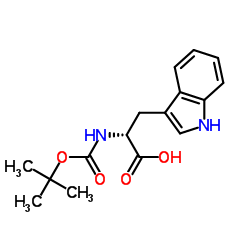
Boc-D-Trp-OH structure
|
Common Name | Boc-D-Trp-OH | ||
|---|---|---|---|---|
| CAS Number | 5241-64-5 | Molecular Weight | 304.341 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 535.7±45.0 °C at 760 mmHg | |
| Molecular Formula | C16H20N2O4 | Melting Point | 131-136ºC | |
| MSDS | Chinese USA | Flash Point | 277.8±28.7 °C | |
| Name | (2R)-3-(1H-indol-3-yl)-2-[(2-methylpropan-2-yl)oxycarbonylamino]propanoic acid |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 535.7±45.0 °C at 760 mmHg |
| Melting Point | 131-136ºC |
| Molecular Formula | C16H20N2O4 |
| Molecular Weight | 304.341 |
| Flash Point | 277.8±28.7 °C |
| Exact Mass | 304.142303 |
| PSA | 91.42000 |
| LogP | 2.89 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.602 |
|
~96% 
Boc-D-Trp-OH CAS#:5241-64-5 |
| Literature: NEUROPORE THERAPIES, INC.; WRASIDLO, Wolfgang Patent: WO2014/14937 A1, 2014 ; Location in patent: Paragraph 0125; 0128 ; |
|
~86% 
Boc-D-Trp-OH CAS#:5241-64-5 |
| Literature: Kim, Sunggak; Chang, Heung Journal of the Chemical Society, Chemical Communications, 1983 , # 22 p. 1357 - 1358 |
| Precursor 3 | |
|---|---|
| DownStream 9 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Oxidative modification of tryptophan residues exposed to peroxynitrite.
Biochem. Biophys. Res. Commun. 234(1) , 82-4, (1997) The aim of this study was to clarify the mechanism of loss of Trp residues in proteins exposed to peroxynitrite. The Trp residues in bovine serum albumin and collagen IV were decreased by peroxynitrit... |
|
|
Abilities of some tryptophan and phenylalanine derivatives to inhibit gastric acid secretion.
Biochim. Biophys. Acta 845(2) , 158-62, (1985) Benzotript (N-p-chlorobenzoyl-L-tryptophan) has been shown to be a receptor-antagonist in vivo and in vitro for peptides from the gastrin family. In the present study, we examine tryptophan, and some ... |
|
|
Methods for determination of electrophoretic mobility and stability of complexes originating in solutions during the chiral discrimination process.
Electrophoresis 19(2) , 276-81, (1998) An equation for the calculation of electrophoretic mobility of kinetically labile complexes originating in solutions during the chiral discrimination process is derived. The mobility of the complex is... |
| N-BOC-L-Tryptophane |
| Nalpha-Boc-D-tryptophan |
| D-N-Boc-trytophan |
| N-Boc-R-tryptophan |
| Boc-D-Trp-OH |
| BOC-D-TRYPTOPHANE |
| MFCD00037944 |
| N-(tert-Butoxycarbonyl)-D-tryptophan |
| EINECS 226-042-1 |
| Boc-D-tryptophan |
| N-{[(2-Methyl-2-propanyl)oxy]carbonyl}-D-tryptophan |
| Nalpha-Boc-D-Tryptophane |
| Boc-D-Trp |
| N-Boc-D-tryptophan |
| D-Tryptophan, N-[(1,1-dimethylethoxy)carbonyl]- |
| tert-butyloxycarbonyl-D-tryptophan |

 CAS#:171596-28-4
CAS#:171596-28-4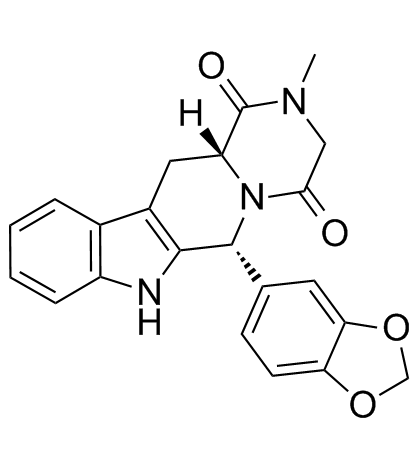 CAS#:171596-29-5
CAS#:171596-29-5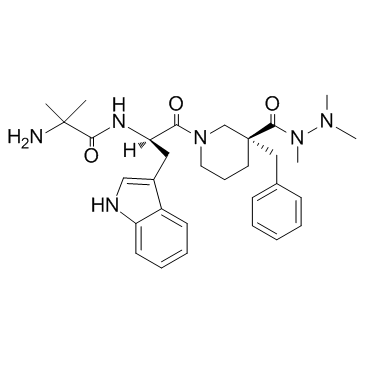 CAS#:249921-19-5
CAS#:249921-19-5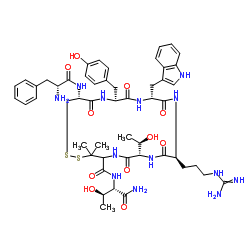 CAS#:103429-32-9
CAS#:103429-32-9 CAS#:103429-31-8
CAS#:103429-31-8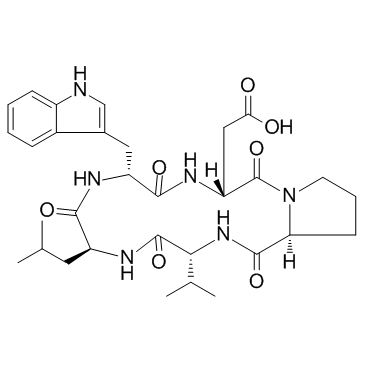 CAS#:136553-81-6
CAS#:136553-81-6 CAS#:158932-00-4
CAS#:158932-00-4 CAS#:159634-94-3
CAS#:159634-94-3 CAS#:81377-02-8
CAS#:81377-02-8
