CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
KH7880000
-
CHEMICAL NAME :
-
Ethane, 2-(o-chlorophenyl)-2-(p-chlorophenyl)-1,1-dichloro-
-
CAS REGISTRY NUMBER :
-
53-19-0
-
BEILSTEIN REFERENCE NO. :
-
2056007
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
25
-
MOLECULAR FORMULA :
-
C14-H10-Cl4
-
MOLECULAR WEIGHT :
-
320.04
-
WISWESSER LINE NOTATION :
-
GYGYR BG&R DG
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
17 gm/kg/35W
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
800 mg/kg/4D
-
TOXIC EFFECTS :
-
Skin and Appendages - dermatitis, other (after systemic exposure)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
11 gm/kg/15W
-
TOXIC EFFECTS :
-
Vascular - BP lowering not characterized in autonomic section Blood - normocytic anemia Blood - pigmented or nucleated red blood cells
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
14 gm/kg/22W
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Gastrointestinal - hypermotility, diarrhea Gastrointestinal - nausea or vomiting
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>4 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
8400 mg/kg/28D-I
-
TOXIC EFFECTS :
-
Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - phosphatases
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
2800 mg/kg/28D-I
-
TOXIC EFFECTS :
-
Endocrine - other changes Endocrine - changes in adrenal weight Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - phosphatases
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
4200 mg/kg/14D-I
-
TOXIC EFFECTS :
-
Endocrine - other changes Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
10 gm/kg/52W-C
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Reproductive - Tumorigenic effects - testicular tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2500 mg/kg/7W-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Blood - lymphoma, including Hodgkin's disease Skin and Appendages - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
9750 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Lungs, Thorax, or Respiration - tumors Blood - lymphoma, including Hodgkin's disease
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
16 gm/kg
-
SEX/DURATION :
-
male 15 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count) Reproductive - Paternal Effects - testes, epididymis, sperm duct
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
250 mg/kg
-
SEX/DURATION :
-
female 15-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - endocrine system Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
900 mg/kg
-
SEX/DURATION :
-
female 6-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
900 mg/kg
-
SEX/DURATION :
-
female 6-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
900 mg/kg
-
SEX/DURATION :
-
female 6-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
MUTATION DATA
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TEST SYSTEM :
-
Rodent - rat Cells - not otherwise specified
-
DOSE/DURATION :
-
10 ug/L
-
REFERENCE :
-
34LXAP "Insecticide Biochemistry and Physiology," Wilkinson, C.F., ed., New York, Plenum Pub. Corp., 1976 Volume(issue)/page/year: -,555,1976 *** REVIEWS *** IARC Cancer Review:Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 53,179,1991 IARC Cancer Review:Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 53,179,1991 IARC Cancer Review:Group 2B IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 53,179,1991 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X2075 No. of Facilities: 10 (estimated) No. of Industries: 1 No. of Occupations: 1 No. of Employees: 197 (estimated) No. of Female Employees: 59 (estimated)
|
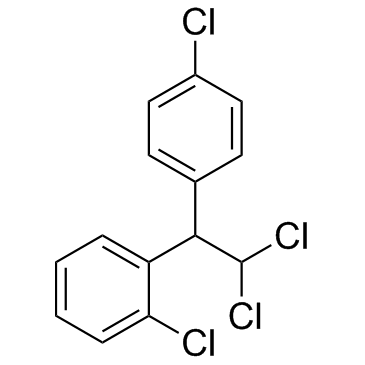


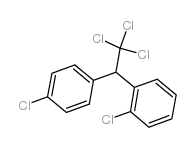 CAS#:789-02-6
CAS#:789-02-6 CAS#:27683-60-9
CAS#:27683-60-9 CAS#:108-90-7
CAS#:108-90-7 CAS#:36692-27-0
CAS#:36692-27-0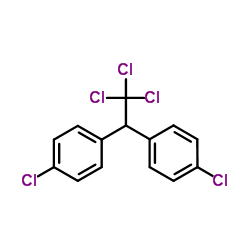 CAS#:50-29-3
CAS#:50-29-3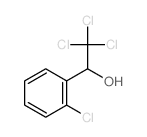 CAS#:10291-39-1
CAS#:10291-39-1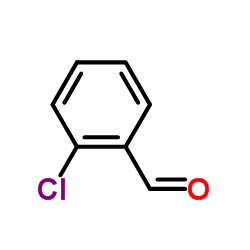 CAS#:89-98-5
CAS#:89-98-5 CAS#:7664-93-9
CAS#:7664-93-9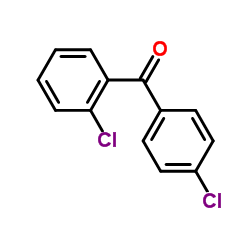 CAS#:85-29-0
CAS#:85-29-0![Benzene,1-chloro-2-[2-chloro-1-(4-chlorophenyl)ethenyl]- structure](https://image.chemsrc.com/caspic/160/14835-94-0.png) CAS#:14835-94-0
CAS#:14835-94-0![Benzene,1-chloro-2-[1-(4-chlorophenyl)ethyl]- structure](https://image.chemsrc.com/caspic/461/77008-62-9.png) CAS#:77008-62-9
CAS#:77008-62-9![1-chloro-2-[1,2-dichloro-1-(4-chlorophenyl)ethyl]benzene structure](https://image.chemsrc.com/caspic/011/90284-72-3.png) CAS#:90284-72-3
CAS#:90284-72-3![Benzene,1-chloro-2-[1-(4-chlorophenyl)ethenyl]- structure](https://image.chemsrc.com/caspic/048/39274-24-3.png) CAS#:39274-24-3
CAS#:39274-24-3![1-chloro-2-[(4-chlorophenyl)methyl]benzene structure](https://image.chemsrc.com/caspic/379/52094-02-7.png) CAS#:52094-02-7
CAS#:52094-02-7
