Androsterone
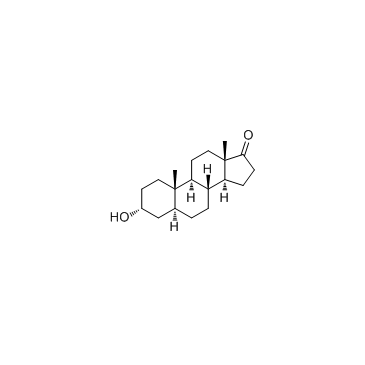
Androsterone structure
|
Common Name | Androsterone | ||
|---|---|---|---|---|
| CAS Number | 53-41-8 | Molecular Weight | 290.440 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 413.1±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H30O2 | Melting Point | 180-185ºC | |
| MSDS | Chinese USA | Flash Point | 176.4±21.3 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of AndrosteroneAndrosterone is a metabolic product of testosterone and can activate Farnesoid X Receptor (FXR). |
| Name | androsterone |
|---|---|
| Synonym | More Synonyms |
| Description | Androsterone is a metabolic product of testosterone and can activate Farnesoid X Receptor (FXR). |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Androsterone activates both the mFXR-LBD and the hFXR-LBD, with Androsterone activating the mFXR-LBD more strongly than the hFXR-LBD. Furthermore, cotransfection studies with gal4-hFXR-LBD and SRC-1/VP16 expression plasmids demonstrate that Androsterone potentiates the interaction of SRC-1 with the hFXR-LBD. Several amino acid changes including H294S, S332V, R351H, and Y361F significantly reduce Androsterone activation[1]. Androsterone (5α, 3α-A) (10 to 100 μM) also inhibits epileptiform discharges in a concentration-dependent fashion in the in vitro slice model[2]. |
| In Vivo | Androsterone treatment results in a significant induction of small heterodimer partner (SHP), suggesting Androsterone may activate endogenous FXR[1]. Intraperitoneal injection of Androsterone (5α, 3α-A) protects mice in a dose-dependent fashion from seizures in the following models (ED50, dose in mg/kg protecting 50% of animals): 6 Hz electrical stimulation (29.1), pentylenetetrazol (43.5), pilocarpine (105), 4-AP (215), and maximal electroshock (224)[2]. |
| Cell Assay | AML-12 cells are used and cultured in a 1:1 mix of DMEM/Ham’s F12 medium supplemented with 10% fetal bovine serum, 1% insulin-transferrin-selenium mix, 40 ng/mL dexamethasone, 100 U/mL penicillin, and 100 μg/mL streptomycin. For gene regulation studies, AML-12 cells are plated in growth medium at 4×105 cells per well in six-well plates. The following day, treatments including Androsterone or dimethylsulfoxide (DMSO) vehicle are added to the medium. At various times after treatment addition, total RNA is prepared. mRNA levels for individual genes are determined by real-time PCR[1]. |
| Animal Admin | Castrated male mice 8 to10 wk of age are fed a casein diet for 2 wk before the start of the study. The mice receive daily sc injections of 0.1 mL 90% corn oil/10% ethanol vehicle or 10 mg/mL Androsterone in vehicle. Animals are killed 2 h after the fifth daily treatment, approximately 4 h after commencement of the light cycle. Total RNA is prepared, and mRNA levels for specific genes are determined by real-time PCR[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 413.1±45.0 °C at 760 mmHg |
| Melting Point | 180-185ºC |
| Molecular Formula | C19H30O2 |
| Molecular Weight | 290.440 |
| Flash Point | 176.4±21.3 °C |
| Exact Mass | 290.224579 |
| PSA | 37.30000 |
| LogP | 3.75 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.536 |
Synonym:3_-Hydroxy-5_-Androstan-17-One; Cis-Androsterone Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: None Listed. Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated. Potential Health Effects Eye: May cause eye irritation. Skin: May cause skin irritation. Ingestion: May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated. Inhalation: May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Chronic: Adverse reproductive effects have been reported in animals. Section 4 - FIRST AID MEASURES Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Skin: Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Ingestion: Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Inhalation: Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Notes to Physician: Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Extinguishing Media: Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation. Section 7 - HANDLING and STORAGE Handling: Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Storage: Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Hormones and antibiotics room. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 53-41-8: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure. Respirators: A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Granules Color: white Odor: None reported. pH: Not available. Vapor Pressure: Not available. Viscosity: Not available. Boiling Point: Not available. Freezing/Melting Point: 181.00 - 184.00 deg C Autoignition Temperature: Not applicable. Flash Point: Not applicable. Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: Solubility in water: practically insoluble Specific Gravity/Density: Molecular Formula: C19H30O2 Molecular Weight: 290.44 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable under normal temperatures and pressures. Conditions to Avoid: Incompatible materials, dust generation, excess heat, strong oxidants. Incompatibilities with Other Materials: Oxidizing agents. Hazardous Decomposition Products: Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide. Hazardous Polymerization: Has not been reported. Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 53-41-8: BV8053000 LD50/LC50: Not available. Carcinogenicity: Androsterone - Not listed by ACGIH, IARC, or NTP. Other: See actual entry in RTECS for complete information. Section 12 - ECOLOGICAL INFORMATION Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. Section 14 - TRANSPORT INFORMATION IATA Not regulated as a hazardous material. IMO Not regulated as a hazardous material. RID/ADR Not regulated as a hazardous material. Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: Not available. Risk Phrases: Safety Phrases: S 24/25 Avoid contact with skin and eyes. S 28A After contact with skin, wash immediately with plenty of water. S 37 Wear suitable gloves. S 45 In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible). WGK (Water Danger/Protection) CAS# 53-41-8: No information available. Canada CAS# 53-41-8 is listed on Canada's DSL List. CAS# 53-41-8 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 53-41-8 is not listed on the TSCA inventory. It is for research and development use only. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H315-H319-H334-H335 |
| Precautionary Statements | P261-P305 + P351 + P338-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38-43 |
| Safety Phrases | 22-24/25 |
| RIDADR | 2811.0 |
| WGK Germany | 3 |
| RTECS | BV8053000 |
| Hazard Class | 6.1 |
| Precursor 9 | |
|---|---|
| DownStream 8 | |
|
Silica-based nanofibers for electrospun ultra-thin layer chromatography.
J. Chromatogr. A. 1364 , 261-70, (2014) Nanofibrous silica-based stationary phases for electrospun ultra-thin layer chromatography (E-UTLC) are described. Nanofibers were produced by electrospinning a solution of silica nanoparticles disper... |
|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
|
|
Next-generation steroidogenesis inhibitors, dutasteride and abiraterone, attenuate but still do not eliminate androgen biosynthesis in 22RV1 cells in vitro
J. Steroid Biochem. Mol. Biol. 144 Pt B , 436-44, (2014) • Dutasteride and abiraterone were evaluated for inhibition of steroidogenesis. • Bypass mechanisms arise in the presence of abiraterone to form DHT. • Dutasteride inhibits T and DHT effectively in vi... |
| 3-α-hydroxy-5α-Androstan-17-one |
| MFCD01317504 |
| Androkinine |
| 5a-Androsterone |
| Androkinin |
| Androtine |
| 3alpha-Hydroxy-5alpha-androstan-17-one |
| Atromide ICI |
| 3-Epihydroxyetioallocholan-17-one |
| (3a,5a)-3-Hydroxyandrostan-17-one |
| (3α,5α)-3-Hydroxyandrostan-17-one |
| (3R,5S,8R,9S,10S,13S,14S)-3-hydroxy-10,13-dimethyl-1,2,3,4,5,6,7,8,9,11,12,14,15,16-tetradecahydrocyclopenta[a]phenanthren-17-one |
| 3α-hydroxy-5α-androstane-17-one |
| Androtin |
| 3alpha-Hydroxyetioallocholan-17-one |
| Androstan-3a-ol-17-one |
| 5alpha-Androsterone |
| 3a-Hydroxyetioallocholan-17-one |
| 5alpha-Androstane-3alpha-ol-17-one |
| Androsterone |
| trans-Androsterone |
| Androstan-17-one, 3-hydroxy-, (3α,5α)- |
| EINECS 200-173-4 |
| Androsterone, trans- |
| Atromide |
| (3α,5α)-3-hydroxy-Androstan-17-one |
| 3a-Hydroxy-5a-androstan-17-one |
 CAS#:846-46-8
CAS#:846-46-8 CAS#:1239-31-2
CAS#:1239-31-2![(3R,5S,8R,9S,10S,13S,14S)-10,13-dimethyl-3-((tetrahydrofuran-2-yl)oxy)tetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one Structure](https://www.chemsrc.com/caspic/085/111222-34-5.png) CAS#:111222-34-5
CAS#:111222-34-5 CAS#:1852-53-5
CAS#:1852-53-5 CAS#:10429-07-9
CAS#:10429-07-9 CAS#:5717-77-1
CAS#:5717-77-1 CAS#:481-29-8
CAS#:481-29-8 CAS#:897-01-8
CAS#:897-01-8 CAS#:58-22-0
CAS#:58-22-0 CAS#:14639-79-3
CAS#:14639-79-3![(5S,8R,9S,10S,13S,14S)-10,13-dimethyl-1,2,5,6,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-one structure](https://www.chemsrc.com/caspic/321/14935-81-0.png) CAS#:14935-81-0
CAS#:14935-81-0 CAS#:1852-41-1
CAS#:1852-41-1 CAS#:1852-43-3
CAS#:1852-43-3 CAS#:66885-58-3
CAS#:66885-58-3
